Collaboration between pharmaceutical giant GSK and Vanderbilt University School of Medicine researcher Neil Osheroff, Ph.D., has given birth to a new antibacterial treatment for Escherichia coli, the most common culprit in uncomplicated urinary tract infections (uUTIs).
“Phase 3 trials for the treatment of uUTIs by gepotidacin, which targets bacterial gyrase/topoisomerase IV, have been halted due to proven efficacy,” Osheroff said. Pending FDA approval, he says gepotidacin could be on the market as soon as 2024. It is also in phase 3 trials for the treatment of uncomplicated urogenital gonorrhea.
“Topoisomerases are the primary target in some of the most active drugs currently used for the treatment of human cancers and bacterial infections, yet we still have much to understand about their mechanism of action,” said Osheroff, who is John G. Coniglio Chair in Biochemistry at Vanderbilt. “Understanding this mechanism has been a decades-old goal of our lab, whether the applications are toward cancer treatment or addressing antibacterial resistance.”
The Resistance Crisis
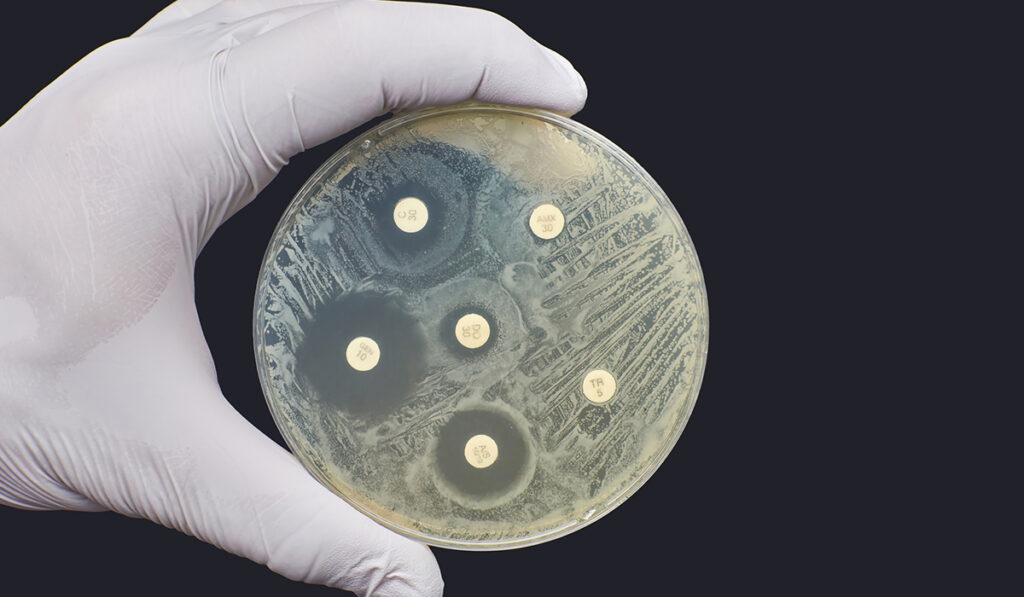
Today, uUTIs largely affect women, with over half developing them and more than a quarter enduring recurrent infections. Broad-spectrum fluoroquinolones like ciprofloxacin, which targets gyrase and topoisomerase IV, have had a considerable tenure of over 40 years in treating uUTIs and other infections. However, fluoroquinolone resistance has grown to over 20 percent in patients with uUTIs and over 50 percent in those with complicated infections.
“There has been a fear that we are going back to a pre-antibiotic era.”
Experts have estimated that deaths due to all forms of antimicrobial resistance are currently around 35,000 per year in the United States, and over one million globally. Global resistance could kill 10 million people annually by 2050, unless new drug classes come to the rescue.
“There has been a fear that we are going back to a pre-antibiotic era. Bacteria are very hard to kill, and they are very good at evading the actions of drugs,” Osheroff said.
Mechanisms of Action
The major classes of current antibacterials have three ways of killing bacteria. Penicillins work by inhibiting cell wall biosynthesis. Tetracyclines work by inhibiting the generation of RNA and proteins.
Fluoroquinolones work by inhibiting the actions of enzymes gyrase and topoisomerase IV.
“These ubiquitous enzymes remove tangles and knots from genetic material and alleviate torsional stress in DNA that accumulates ahead of replication forks and transcription complexes,” Osheroff explained. “Because of their actions, these enzymes play critical roles in DNA replication, transcription and chromosome segregation in all cells.”
Fluoroquinolones act in an insidious manner. In addition to inhibiting overall enzyme activity, they trap enzyme intermediates, fragmenting the genome.
“By this means, fluoroquinolones convert these very important enzymes into toxins that are lethal to cells,” Osheroff said.
Balanced Dual Targeting
Unfortunately, fluoroquinolone effectiveness has been dampened by the evolution of mutations that impair the drug-enzyme interactions. A single mutation in just one of the two enzymes, depending on the species, can render the drug ineffective, Osheroff said.
Gepotidacin evades this weakness because it can kill E. coli cells by targeting either enzyme.
Because of this, “there would have to be simultaneous independent mutations in both gyrase and topoisomerase IV in order for cells to become resistant,” Osheroff said. “This balanced dual-targeting makes the generation of resistance much less likely.”
Osheroff said he gives GSK, the London-based pharmaceutical company, much credit for pursuing the research.
“They went through compound after compound to identify a lead that does not have a negative impact on some aspect of human health.”
“Balanced dual-targeting makes the generation of resistance much less likely.”
Osheroff also praised his lab members, pharmacologist Elizabeth Gibson, Pharm.D., Ph.D., and biochemist Alexandria Oviatt, Ph.D., for their contributions to the team’s delivery of a detailed scientific description of the how the drugs act. GFK is using the data they generated as part of their application to the FDA.
His said his next collaboration with GSK will focus on the interactions of gepotidacin with gyrase and topoisomerase IV in Neisseria gonorrhoeae, the causative agent of gonorrhea and a major cause of pelvic inflammatory disease and infertility.
While fluoroquinolones have had a long run, gepotidacin is projected to have a much longer lifespan, Osheroff said.
“For years I have struggled to try to trace a direct line from research in my lab to human health. I cannot tell you how thrilled we are to see this happen.”