One in 100 women giving birth faces news that the fetus or newborn has congenital heart disease. The physician-family discussions that follow can prompt many different reactions, depending on the predicted postnatal surgeries and outcomes.
To help improve predictions about fetuses with coarctation of the aorta—who may also have a small or hypoplastic left ventricle—a research team at Vanderbilt University Medical Center studied the association between fetal mitral valve size and infants’ postnatal surgical procedures. The work was led by principal investigator Stacy S. Killen, MD, MSCI, co-director of the Fetal Cardiology Program.
“News that your baby has severe congenital heart disease can negatively affect the developing maternal-infant bond,” Killen said. “It’s important to prepare families as best we can for this scenario, if that’s what they truly face, but we want to spare families from distressing ‘false positive’ diagnoses.”
The investigation into this gray zone revealed that, in many cases, outcomes are less dire than historical reasoning has predicted.
“It’s important to prepare families as best we can for this scenario, if that’s what they truly face, but we want to spare what families we can from distressing ‘false positive’ diagnoses.”
Mitral Valve as Bellwether
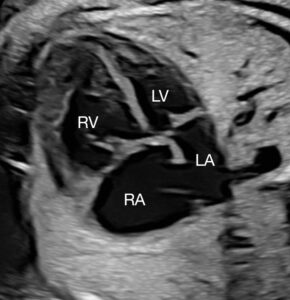
About 8% of fetuses with congenital heart disease have coarctation of the aorta and are investigated for associated aortic valve stenosis, mitral valve stenosis or hypoplastic left heart syndrome. The majority of newborns with prenatally diagnosed coarctation of the aorta have just one cardiac surgery (aortic arch repair) with a surgical mortality less than 2%. Those who have severe left heart obstruction, however, require single-ventricle palliation, including multiple cardiac surgeries, potentially up to heart transplantation. Killen says these patients face a five-year survival rate of about 75%.
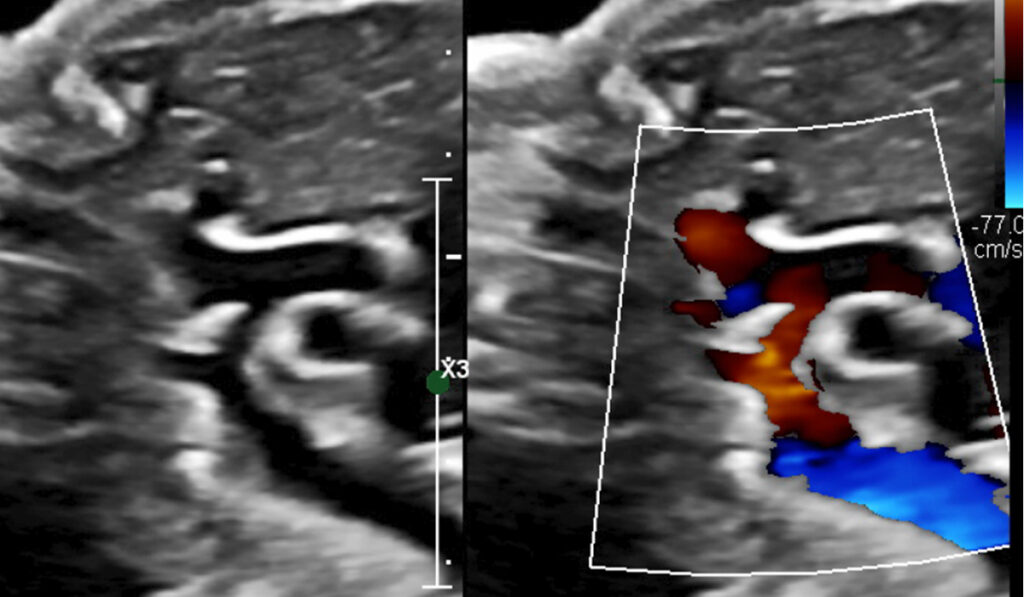
Prenatal diagnosis of coarctation of the aorta through fetal echocardiography is tricky, with the ductus arteriosus often interfering with identification of the coarctation. Taking measurements and drawing conclusions about associated left-heart hypoplasia is complicated by numerous factors. A small mitral valve accompanied by a small left ventricle that is long and narrow may ultimately place the infant in the normal range after birth, while short, wide left ventricles are often inadequate for a biventricular course postnatally.
Raising the Severity Threshold
The team conducted a retrospective review from 2010 to 2020 of 67 fetuses with postnatally confirmed coarctation of the aorta, excluding those with other major congenital heart defects. They used fetal ultrasound to measure the mitral valves and compared the results between groups with postnatal biventricular repair versus single-ventricle palliation, death or transplantation outcomes.
Prenatally there were 43 patients (69%) in the biventricular group with abnormal mitral valves, including 41 with hypoplastic annulus measurements (z-score less than −2.0).
After birth, 62 of the 67 infants were able to undergo a biventricular repair.
Significantly, all five infants who required single-ventricle palliation, as well as those who died or were referred for transplant, had hypoplastic mitral valve z-scores below -4.0.
The researchers also looked at measurements of the left ventricle, aortic arch and aortic valve and the direction of flow across the foramen ovale. Of all of these, however, the size of the mitral valve was found to have the highest correlation with the postnatal surgical course, though at a different threshold than historically presumed.
“As expected, a severely small mitral valve predicted poorer odds of having just one cardiac surgery,” Killen said. “But the data also suggested that the threshold for ‘borderline hypoplasia’ should be raised, meaning we can offer a probability of brighter prospects when fetuses have a mitral valve Z-score of -3.0 or larger.”
Predictions Impact Resources
Killen expects that as ultrasound technology continues to evolve, visibility of the heart structures, including the coarctation of the aorta, will continue to improve outcome predictions.
“Just in the past few years, advances have enabled us to more clearly evaluate valve morphologies in utero, for example, but it is still challenging to adequately image all of the heart structures,” she said.
Through improved ultrasound technology and collaborative multicenter studies on outcomes, Killen expects to see increasingly refined predictions.
“Not only is it important for families’ emotional readiness, but for other preparations as well. When we know complex surgeries are going to be needed, the baby needs to be delivered at a tertiary center,” Killen said. “If their heart is normal or only requires a straightforward repair, they can deliver close to home and not disrupt the normal plans for the family.”
“All of these things can make a world of difference.”