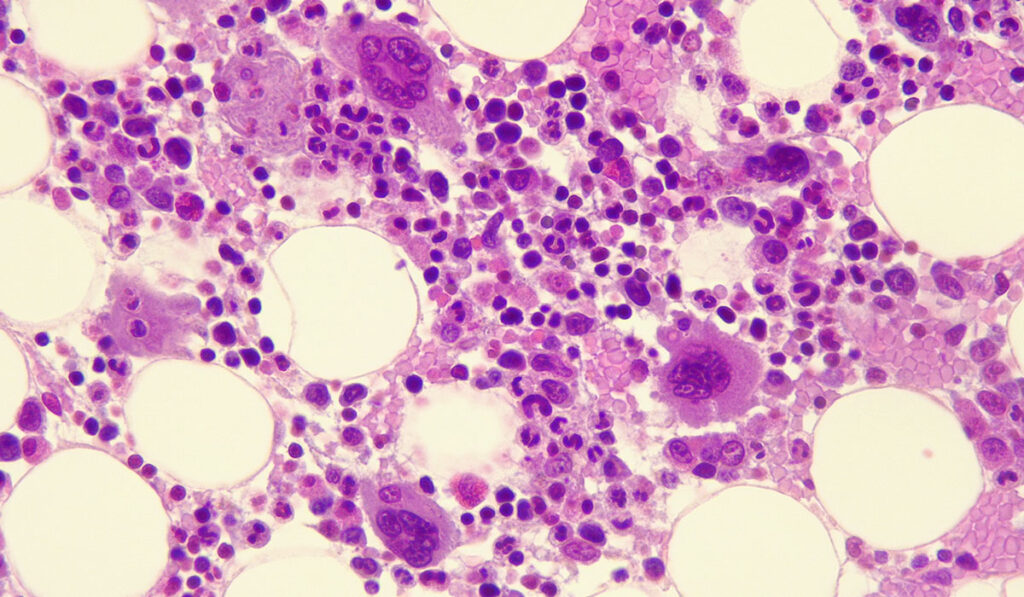
With limited therapies capable of prolonging survival for myelodysplastic syndrome (MDS) patients, investigators at Vanderbilt University Medical Center are weighing the impact of an alternative approach, especially in cases involving TP53 mutations.
These mutations have been recognized as an independent prognostic factor for poor survival and outcomes, especially compared to those with wild-type TP53 status.
The Vanderbilt team was part of a nationwide investigation of ASTX727, an oral, fixed-dose combination of decitabine and the cytidine deaminase inhibitor cedazuridine, that has pharmacokinetic equivalence to standard intravenous decitabine. They examined rates of overall survival (OS) and leukemia free-survival (LKF), as well as evidence of TP53 mutation.
The new results indicated the potential utility of this oral hypomethylating agent in patients with MDS harboring a TP53 mutation.
“These data support considerable therapeutic utility of oral ASTX727 in the treatment of patients with MDS,” said Michael R. Savona, M.D., a professor of medicine and cancer biology at Vanderbilt whose research focuses on novel therapeutic approaches to MDS. He led the work and presented the results at the American Society of Hematology Annual Meeting in December 2022 in New Orleans.
The ASCERTAIN Trial
This post-hoc analysis of the ASCERTAIN trial examined the mutational profile of patients and its impact on key treatment outcomes, including OS and LFS. In the study, the population of patients harboring a TP53 mutation (44 of 125 patients) was characterized by allelic status: 14 had biallelic mutations and 30 had monoallelic mutations lacking other chromosomal deletions.
The median OS was 25.5 months in patients with MDS with a TP53 mutation treated with ASTX727, compared to 33.7 months in patients with wild-type TP53 status.
When looking at allelic status, the median OS in patients with biallelic mutations treated with ASTX727 was 13.0 months verses 29.2 in those with monoallelic mutations, emphasizing the impact of TP53 mutation on survival in MDS.
“MDS patients with mutated TP53 are of particular interest, given the typically poor outcomes and lack of suitable treatment,” Savona said. “This analysis supports the emerging hypothesis that a higher burden of mutant TP53 cells denotes poorer risk, but also reveals that the fixed-dose combination of ASTX727 may serve as a reasonable option in these patients, given the dismal expected survival with previous standards of care.”
“Higher burden of mutant TP53 cells denotes poorer risk, but the fixed-dose combination of ASTX727 may serve as a reasonable option in these patients.”
Unmet Need Remains
Despite these promising results, a significant unmet need remains for patients with MDS, particularly for those with mutations that typically don’t respond well to existing treatment, which is why Savona and his team were encouraged by what they saw in ASCERTAIN.
“Oral delivery of decitabine alleviates the significant burden of five days of monthly IV infusions for patients, who may continue to benefit from the drug for several months or even years,” Savona said.
He also acknowledged that the study had limitations. Notably, the limited number of patients with TP53 mutations and the post-hoc nature of the analysis constrain the generalizability of the findings. Nevertheless, they provide important information in a growing body of literature showing the association between ASTX727 and improved survival outcomes, Savona said.
Next Steps
Savona and his team are conducting additional research to assess the utility of this novel oral therapy as a treatment option for MDS patients. Investigations of combinations of ASTX727 with other novel agents are also being led by Vanderbilt researchers in ongoing trials.
Savona hopes that these results will help increase awareness of available treatments among patients and within the medical oncology community.