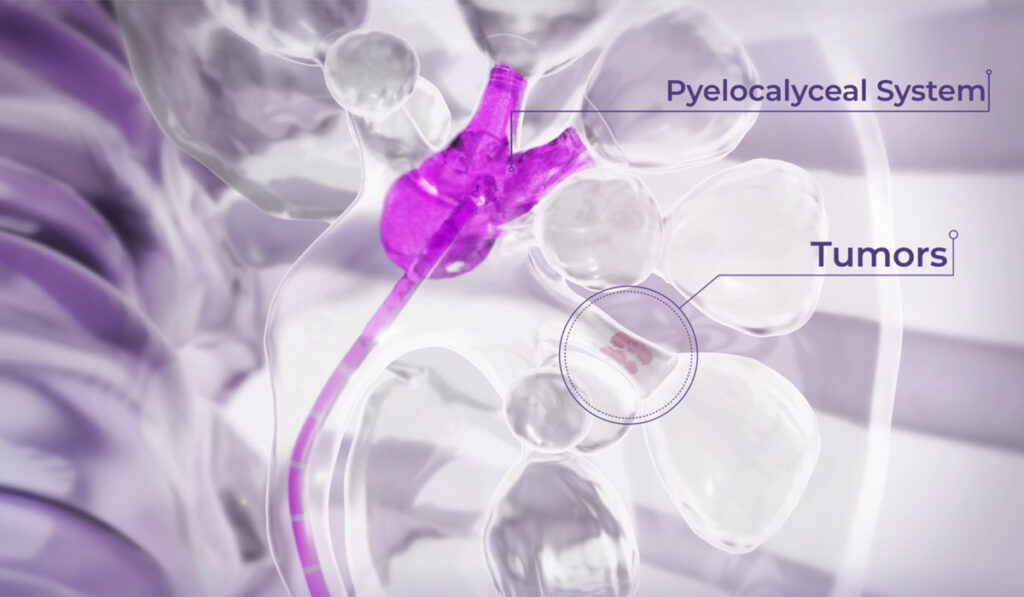
Until now, there has been a clear, unmet need for an effective treatment for patients who have upper tract urothelial cancers, one that would be confined to the kidney itself, rather than requiring the removal of a kidney and ureter.
“Kidney cancer isn’t like bladder cancer where we can instill a fluid that contains chemo drugs into the bladder and ask a patient to hold it there,” said Amy Luckenbaugh, M.D., an assistant professor of urology at Vanderbilt University Medical Center.
The key innovation underlying the new approach involves introducing mitomycin into the kidney by means of a specially formulated solution that is liquid at room temperature and becomes a gel when it warms to body temperature.
“The way that it turns from a liquid into a gel allows it to sit in the kidney and provides that dwell time, which is so important in treating this form of cancer,” Luckenbaugh said.
Mitomycin gel represents the first FDA-approved treatment for upper tract urothelial cancers. It is being marketed as Jelmyto by UroGen Pharma, a firm headquartered in New Jersey, with operations in Israel.
Few Effective Options Before
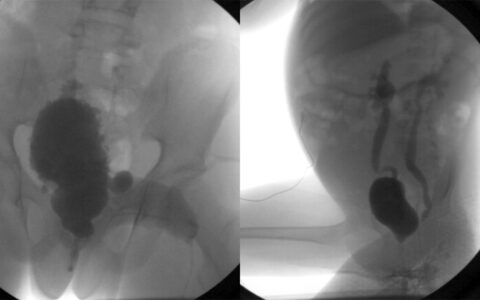
Before the approval of mitomycin gel, urological surgeons had two options for treating upper tract urothelial cancers, Luckenbaugh explained. “One approach was that we would look up the ureter tube with a small camera and introduce a fine laser fiber to burn all the tumor we could see.”
Visualizing these cancers, which often resemble coral or cauliflower, often proves difficult. “As you laser it, it starts to bleed, and then it’s hard to see,” she said.
The second approach, with large tumors, has involved surgically removing the entire kidney and ureter. “This approach was especially problematic in people who had only one kidney or for patients at risk of developing cancer again on the opposite side,” she said.
Useful for Certain Patients
Although urothelial cancer is the tenth most commonly diagnosed cancer worldwide, with approximately 573,000 cases per year, only a particular subset of patients qualify for the new treatment.
Candidates for the treatment must have cancer that is low-grade and confined to the kidney lining; it cannot have spread to the ureter tube.
“That means that the gel treatment works for a small subset of a small subset of patients. For those for whom it is appropriate, it’s very good,” Luckenbaugh said.
Each patient undergoes treatment once a week for six weeks.
“They come in weekly and get an instillation; it’s similar to what we do for bladder cancer,” Luckenbaugh said.
Sparing Patients Dialysis
The mitomycin gel treatment is less invasive than surgical excision of the kidney and ureter and allows preservation of the organ. This kind of less-invasive approach is especially valuable in the realm of upper tract urothelial cancers because many people with the disease are older and have comorbidities.
Right now, its use has been limited to low-grade upper tract urothelial carcinoma; however, it has been used off-label, for high-grade cancers.
“I’ve used it for patients who have a solitary kidney and a high-grade tumor which keeps coming back,” Luckenbaugh said. “It’s hard to remove the remaining kidney and put a patient on dialysis.”