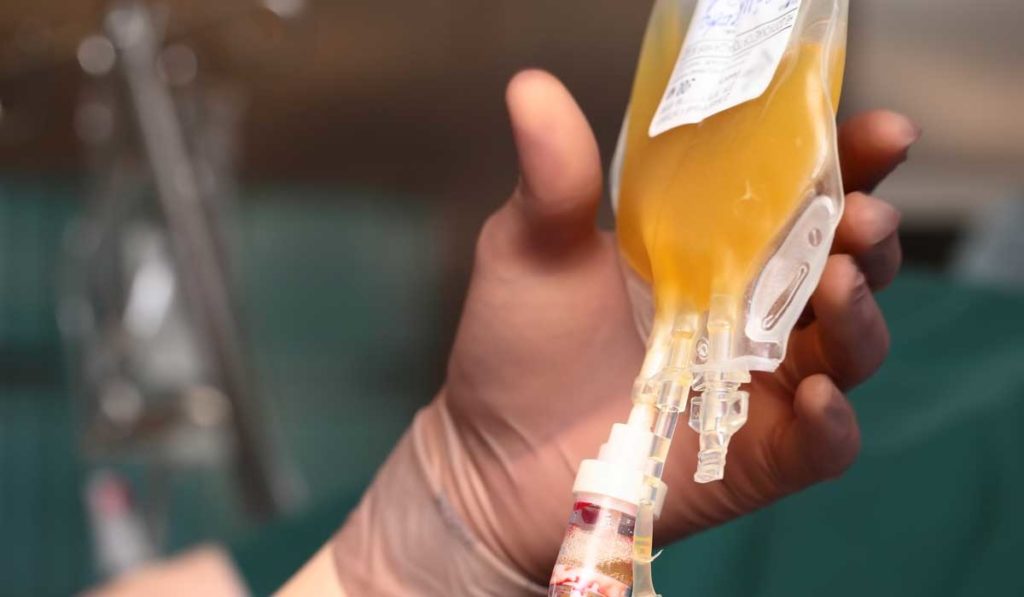
Vanderbilt University Medical Center has launched a randomized controlled trial, the Passive Immunity Trial of Nashville (PassItOn), aimed at strengthening understanding of how convalescent plasma may be effective at treating COVID-19. PassItOn is one of only a few convalescent plasma trials for COVID-19 that are placebo-controlled, says Todd Rice, M.D., associate professor of medicine at Vanderbilt and principal investigator of the trial’s treatment arm.
“Most of the trials are just open-label – giving plasma, collecting data. In order to figure out if the plasma really is working, we need a control arm,” Rice said.
If the therapeutic response is promising, the PassItOn investigators intend to examine more nuanced questions about timing and dosage.
“If you look at the other trials that are going on, like the Expanded Access Program, they’re transfusing convalescent plasma based on a number of assumptions,” said One of our goals is to learn more about the viral response, both to people who recovered as well as to those who are sick and may benefit from convalescent plasma.”
“They’re transfusing convalescent plasma based on a number of assumptions… We’re working with a research-based assay.”
The Collection Arm
The first phase of the PassItOn trial involves recruiting volunteers to donate plasma who have tested positive for COVID-19 and have fully recovered. The donation is being run by a donor agency called Blood Assurance located in Chattanooga, Tennessee.
The inclusion criteria include volunteers who tested positive for COVID-19, recovered, and are at least 14 days from a negative COVID-19 test; patients who tested positive and are at least 28 days free of symptoms but have not had a negative COVID-19 test; and potentially, patients who had clinical symptoms but have never been tested for COVID-19. They will be tested to confirm they have been exposed to the virus.
Additionally, volunteers will need to meet the general FDA guidelines on blood donation to screen for infectious diseases and risk factors. “We’re thinking about the safety of the volunteer, and also the safety of the staff that’s going to be collecting the blood products,” said Wheeler.
The Treatment Arm
The second phase of the program is a randomized, partially double-blinded, placebo-controlled trial for hospitalized adults with laboratory-confirmed COVID-19 and less than 14 days of symptoms.
Patients in the treatment arm will receive a single dose of pathogen reduced SARS-CoV-2 convalescent plasma. The placebo arm will receive Lactated Ringer’s solution with multivitamins. “We spent a lot of time talking about what the control arm should be,” Rice said. “What can we have that will look somewhat like plasma, but that won’t have an effect in the patient?”
Rice explained that Lactated Ringer’s with multivitamins is essentially a crystalloid with some vitamins to make the solution turn a yellow color similar to plasma so that people won’t be able to tell what it is. “It’s not perfect,” he added. “There are some differences in viscosity and consistency. But the patients don’t know, the providing team doesn’t know, and we in the study group don’t know, which is the important part.”
The goal is to enroll 500 patients across multiple centers.
Research to Inform the Guidelines
The general concept of convalescent plasma therapy is well established, says Rice. “Through antibodies present in the plasma, we try and pass the immunity from somebody who’s recovered to somebody who has the disease to help them mount a robust immune response,” he said.
However, no clear guidelines exist regarding the most effective window for plasma collection and transfusion or the target dosage. The PassItOn investigators hope to address these unanswered questions.
“When they come in to donate, how do we know they’ve got the antibodies by that time? Do they have enough antibodies? And are we measuring titers of an antibody that neutralizes the virus? Those are the kinds of questions we’ll investigate,” Rice said.
“When they come in to donate, how do we know they’ve got the antibodies by that time? Do they have enough antibodies? And are we measuring titers of an antibody that neutralizes the virus?”