Immune checkpoint inhibitors (ICIs) can produce long-lasting responses in patients with advanced cancer. In rare cases, however, sudden and fatal autoinflammatory immune-related adverse effects (irAEs) can occur.
In an effort to understand what triggers adverse reactions to ICIs, and to identify susceptible patient populations, a team of investigators at the Vanderbilt-Ingram Cancer Center have been chronicling ICI toxicities.
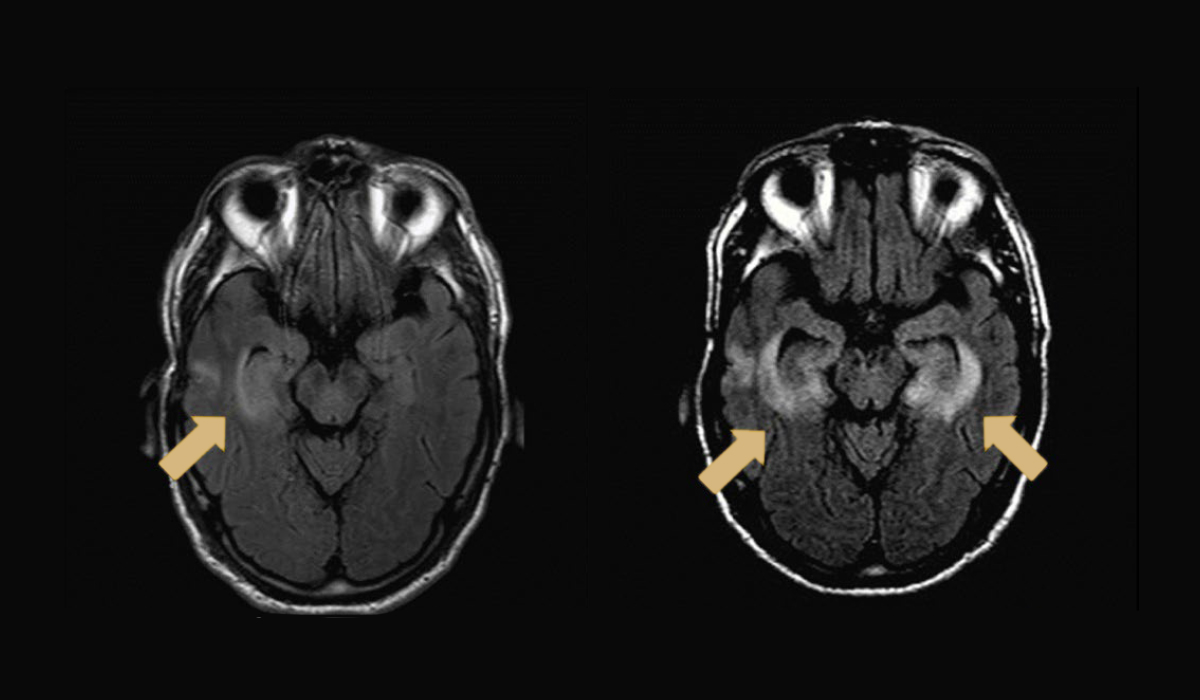
In a recent study published in Nature Medicine the team identified CD4+ and CD8+ T cells as culprits of checkpoint inhibitor-associated immune encephalitis. While cases are rare, encephalitis is the most common neurological toxicity associated with ICI therapy.
“These cases are incredibly rare, but they are significant enough because these drugs are being used much more frequently in an increasing number of cancer types,” said Justin Balko, Ph.D., assistant professor of medicine at Vanderbilt University Medical Center and lead investigator on the study.
A Surprising Role for CD4+ T ‘helper’ cells
The investigators performed deep molecular profiling of a fatal case of anti-programmed cell death receptor 1 (PD-1)-induced encephalitis. The multi-omics approach revealed a population of clonal cytotoxic CD4+ T cells in affected brain tissue and central nervous infiltration by both CD4+ and CD8+ T cells. The role of CD4+ ‘helper’ T cells rather than CD8+ ‘killer’ T cells was a surprising discovery.
“We tend to think of CD8 positive T cells as being more important for an immune therapy side effect,” explained Douglas Johnson, M.D., assistant professor in Vanderbilt’s Division of Hematology and Oncology and first author on the study. “Showing a CD4 positive link is interesting and important.”
“CD4 positive T cells can really play a major role, not just in toxicity, but also in anti-tumor responses.”
Added Balko, “The field is starting to recognize more that these CD4 positive T cells can really play a major role, not just in toxicity, but also in anti-tumor responses.”
Involvement of Viral Infection
Molecular analysis of affected brain tissue also revealed the presence of Epstein-Barr-virus-specific T cell receptors and Epstein-Barr positive lymphocytes, providing evidence of concurrent infection with Epstein-Barr virus. The investigators believe this may be the first molecular report linking ICI-induced neurotoxicity with a viral infection.
“While this encephalitis case seemed to be associated with some sort of viral process, it is still very unclear how often this happens,” Johnson said. “I think there is more research that needs to be done to tease out the role of viral infection as it relates to immune therapy side effects.”
Scope of ICI-induced Encephalitis
In order to determine the frequency of ICI-induced encephalitis, the investigators turned to data mining.
Query of global pharmacovigilance databases identified 209 cases of encephalitis among patients treated with ICIs; 59 percent had either melanoma or lung cancer, 66 percent received anti-PD-1 monotherapy, 88 percent had no other reported irAEs and 19 percent died.
The results indicate that encephalitis is a rare but recurrent and potentially fatal toxicity of ICIs that occurs across cancer types. Balko says additional studies are needed to determine preventative measures and management strategies for these rare but severe irAEs.