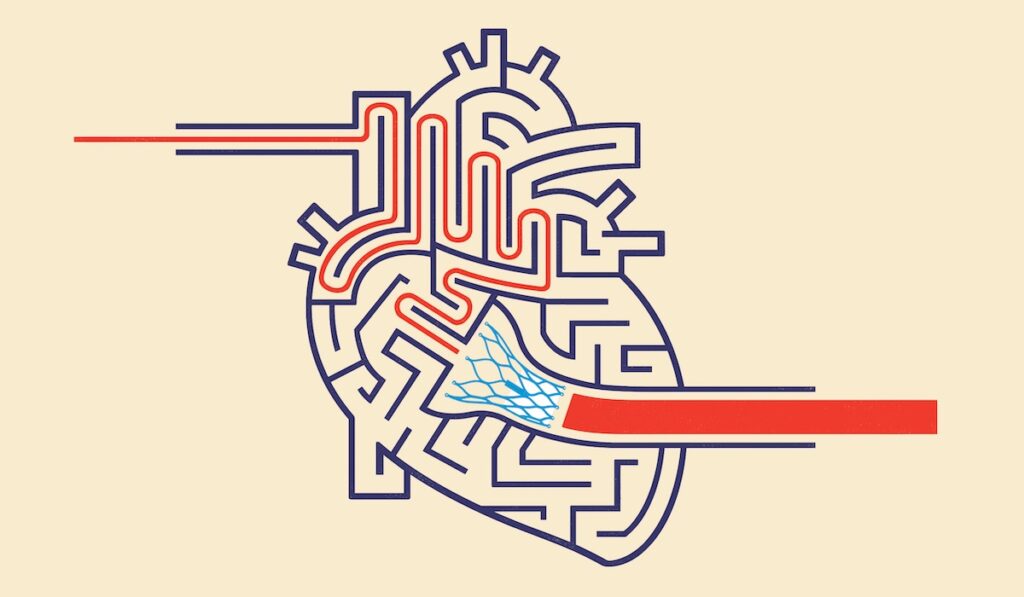
The results of the EARLY TAVR trial, published in the New England Journal of Medicine, showed that prompt treatment with transcatheter aortic valve replacement is superior to routine clinical surveillance in patients with asymptomatic severe aortic stenosis. Based on these findings, TAVR using the Edwards Sapien valve has been approved for treating asymptomatic severe AS.
Numerous randomized trials over the last decade have expanded the indication of TAVR— initially an option only for patients considered extreme risk or high risk for surgery—to include symptomatic patients of all risk levels, said Brian R. Lindman, MD, an associate professor of medicine and medical director of Vanderbilt Health Structural Heart and Valve.
“With mature devices and very safe procedures, we are now working to identify new patient populations that might benefit from intervention — and patients with asymptomatic severe AS appear to be one such population,” Lindman said.
Tarek S. Absi, MD, associate professor of cardiac surgery at Vanderbilt, recognizes this shift in the paradigm.
“Patient eligibility for TAVR continues to expand,” Absi said. “It’s become a popular option, even in low-risk populations where treatment decisions are made collaboratively and are much more nuanced.”
Interpreting the Trial Results
Prior to EARLY TAVR, two randomized trials (RECOVERY; AVATAR) demonstrated the benefits of early intervention with surgical aortic valve replacement in patients with asymptomatic severe AS. However, small sample sizes and a limited number of events driving the results yielded uncertainty with respect to the strength of the conclusions, Lindman said.
EARLY TAVR was the first and largest study to test a transcatheter aortic valve intervention for patients with asymptomatic severe aortic stenosis.
The study examined 901 patients with severe aortic stenosis whose lack of symptoms was verified by a normal treadmill stress test. Those who received early intervention with TAVR did better in the long run than those who underwent clinical surveillance until symptoms triggered valve replacement.
These results were consistent for the primary endpoint—a composite of death, stroke or unplanned hospitalization for cardiovascular causes—and several secondary endpoints. Additionally, there were no apparent differences in procedure-related adverse events between patients who received TAVR and those who underwent clinical surveillance with deferred valve replacement.
“Taken together, the results pointed to the benefit of an early intervention strategy with TAVR,” Lindman said.
“Taken together, the results pointed to the benefit of an early intervention strategy with TAVR.”
The Biomarker Question
A unique aspect of the EARLY TAVR trial was its prospective collection of biospecimens, enabling a prespecified cardiac biomarker analysis to determine whether a subgroup of patients could potentially benefit from early aortic valve replacement.
Vanderbilt University Medical Center was the biobank core laboratory for the trial, and the results of the primary analysis were recently published in Circulation.
Lindman, first author on the publication, and colleagues hypothesized that patients with elevated biomarker levels would have higher event rates and would experience greater benefit from early TAVR compared with those with lower biomarker levels. Now, a societal guideline is based on this hypothesis.
To their surprise, there was no differential treatment effect of early TAVR compared with clinical surveillance based on baseline biomarker levels.
“Whether biomarker levels were high or low, patients still derived benefit from early intervention versus clinical surveillance,” Lindman said. “As such, there appears to be limited value to a single biomarker assessment to guide treatment timing in patients with asymptomatic severe AS. However, biomarkers may serve to guide timing of intervention in patients with moderate AS, which will be tested in the forthcoming PROGRESS trial.”
Emerging Strategies
Lindman, Absi and colleagues also are exploring the potential utility of early TAVR in different patient populations, such as those with moderate aortic stenosis.
For example, the PROGRESS trial is evaluating whether prompt intervention with TAVR yields better outcomes than clinical surveillance with deferred valve replacement for moderate aortic stenosis patients with symptoms or other high-risk features.
While the randomized portion of the trial has been completed, patients can still enroll at Vanderbilt and other trial sites in the expanded access component of the study, Lindman explained. If they meet the inclusion criteria, they can still receive valve replacement without randomization.
“At Vanderbilt, patients can access treatments that are not otherwise commercially available,” Lindman said. “The PROGRESS trial is a prime example.”
To date, the focus of almost all trials and commercial treatment has been on transcatheter treatment of aortic stenosis. Recently, Vanderbilt became an enrolling site in the JOURNEY trial to evaluate the safety and efficacy of a new transcatheter valve replacement system in patients with aortic regurgitation at high-risk for surgery.
“Vanderbilt’s multidisciplinary heart team can assist patients in navigating the numerous clinical trial and commercial options we have available,” Absi said. “A multidisciplinary valve team with input from interventionalists, surgeons and cardiac-imaging experts allows us to offer the best treatment options to patients to optimize their quantity and quality of life. This is an exciting time for the treatment of patients with aortic valve disease, including both AS and aortic regurgitation.”
“Vanderbilt’s multidisciplinary heart team can assist patients in navigating the numerous clinical trial options we have available.”