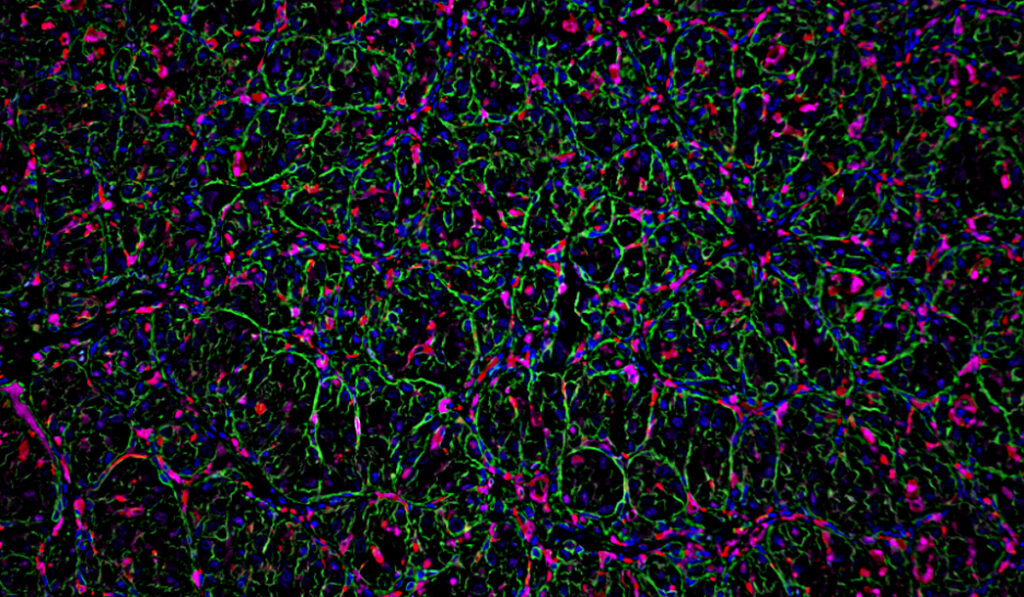
Cancer treatments build, in large part, on learnings that span multiple tumor types, but in clear cell renal cell carcinoma (ccRCC), the most common type of kidney cancer, research is complicated by unique factors in the tumor microenvironment, ones that markedly differ from those of other cancers.
At Vanderbilt University Medical Center, Jeffrey Rathmell, Ph.D., director of the Vanderbilt Center for Immunobiology at VUMC, and Kimryn Rathmell, M.D., Ph.D., former Hugh Jackson Morgan Professor and Chair of Medicine at Vanderbilt and now director of the National Cancer Institute, recently co-mentored Melissa Wolf, Ph.D., on her dissertation project. Her aim was to learn how a loss of function of the von Hippel Lindau (VHL) gene in patients with ccRCC impacts immune cell infiltration and function in the tumor microenvironment.
“Melissa identified how the tumor microenvironment changes when VHL is lost and, in that process, discovered a potential new target in ccRCC,” said Jeffrey Rathmell.
That target is CX3CL1, a cytokine and signaling protein that is upregulated in cells where VHL is inactivated. Wolf’s experiments showed that a loss of VHL, and subsequent upregulation of CX3CL1, promote an increase of proinflammatory macrophages around tumors. The team published their findings in The Journal of Clinical Investigation.
“Only recently have we started to appreciate the role of cancer cell genetics on the tumor microenvironment in this cancer,” Wolf said. “This work recognizes the role of a specific gene loss and reveals a previously unknown way in which cancer cells affect the function of immune cells in the immediate region of the tumor.”
“This work reveals a previously unknown way in which cancer cells impact the function of immune cells in the immediate region of the tumor.”
Dysregulated Hypoxia Response
Clear cell renal cell carcinoma represents between 66-75 percent of all kidney cancer diagnoses. Loss of the VHL gene, a truncal mutation in ccRCC, is one notable example of how ccRCC differs from other cancers.
“Unlike in melanoma or lung cancer, where a high infiltration of immune cells is thought to be initiated by neoantigens caused by carcinogens like smoking or UV exposure, we don’t know the exact causes of the infiltration in ccRCC,” Wolf said. “What we do know is that 75- to 80-percent of patients who develop ccRCC have lost the VHL gene, leading to dysregulation of the hypoxia response pathway.”
VHL senses oxygen levels. In a healthy cell, when oxygen levels are low, VHL will turn on the hypoxia response pathway by upregulating the production of hypoxia-inducible factors (HIFs), Wolf said. But VHL loss disrupts the cell’s ability to make these adaptations. With VHL lost, HIFs are continually upregulated, and the loss of checks and balances supports tumor growth.
Immune Cell Indoctrination
Wolf used mouse models of kidney cancer to study the effects of VHL loss compared to wildtype VHL in the tumor microenvironment. Her study demonstrated that the myeloid compartment in the microenvironment functioned differently in the VHL-deficient group, where myeloid cells were more inflammatory, and both myeloid and CD4+ helper T cells were more abundant.
In addition, targeting T cells slowed tumor growth in the VHL wildtype group but had little impact in the VHL knockout tumors.
Prominent Role of CX3CL1
Wolf also found an association of VHL loss with the upregulation of the chemoattractant CX3CL1, important in broadly recruiting immune cells but specifically inflammatory myeloid cells to the tumor.
Counterintuitively, when VHL was knocked out, tumors grew more slowly. In a double knockout of both the VHL and CX3CL1 genes, tumor growth was considerably slower still.
Wolf posits that CX3CL1 upregulation promotes the migration of myeloid immune cells to the tumor site. Once there, rather than attacking the tumor, these cells may be enlisted by the tumor to evade attack from other immune cells.
Current Treatment Shortfalls
Immunotherapy drugs commonly prescribed for ccRCC include checkpoint inhibitors of T cell ligands PD-1 and CTLA-4, which block the T cells’ ability to perform their immune function. Other treatment paradigms use drugs to either block HIF or the downstream targets with oral tyrosine kinase inhibitors.
Kathryn Beckermann, M.D., Ph.D., an assistant professor of cell and developmental biology at Vanderbilt and co-author on the paper, says that the mechanisms for these drugs are not well-understood, nor are the ratios and dosages standardized.
“Currently, checkpoint inhibitors are prescribed in stage IV ccRCC to try to enable T cell function, but in this complex tumor microenvironment, it is still, unfortunately, a minority of patients who experience durable benefit from these therapies,” she said. “Anti-angiogenics tyrosine kinase inhibitors also have great efficacy, but the tumor figures out a way to become resistant to these drugs.”
Targeting Potential
“We don’t know exactly how to connect the dots between VHL loss, HIF, and CX3CL1 upregulation and the infiltration of immune cells, but we see these strong associations,” Wolf said. “Targeting CX3CL1 in the setting of VHL loss may lead to slower growing tumors – we will just need to test it.”
Toward such interventions, the researchers are continuing work to decipher targets that help regulate immune cell function. Identifying CX3CL1’s role may be a leap toward developing more effective interventions.