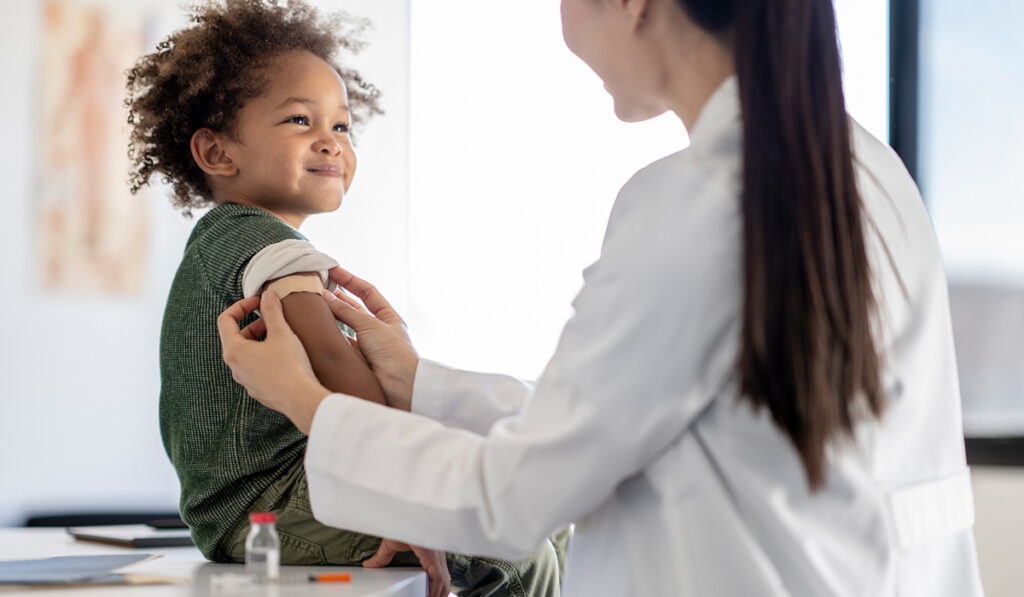
Pediatric transplant patients are at risk of experiencing severe outcomes from respiratory infections – and are more likely to contract them – due to their overall health and the use of immunosuppression therapy.
Pediatric infectious diseases researchers at Vanderbilt University Medical Center recently examined the incidence of influenza infections, as well as the effects of vaccines in pediatric transplant patients.
Their retrospective study, published in the American Journal of Transplantation, looked at influenza incidence in solid-organ transplant cases to help guide future prevention and intervention efforts, said the study’s senior author, Daniel E. Dulek, M.D., an assistant professor of pediatrics and director of the Immunocompromised Host Pediatric Infectious Diseases Program, a dedicated clinical service offered at Vanderbilt.
Incidence and Prevention
Analyzing data on nearly 8,000 pediatric solid-organ recipients over a 10-year period, 2006-2016, Dulek’s team found 2.7 percent of patients had an influenza-associated hospital encounter at one-year post-transplant, with 0.3 percent having incidents that were considered severe. At three years post-transplant, all associated incidents stood at 7.3 percent, with severe incidents at 0.9 percent.
Working alongside principal investigator Dulek was site investigator Natasha B. Halasa, M.D., Craig Weaver Professor of Pediatrics, Infectious Diseases at Vanderbilt. The pair studied high-dose influenza vaccines in transplant patients.
As described in a recent letter to the New England Journal of Medicine, a higher dose – four times the vaccine antigen dose – was initially approved for patients 65 and older.
Vaccine Dosage
Halasa’s team conducted phase 1 studies involving pediatric solid organ transplant patients, adult stem cell transplant patients, and in children with acute lymphoblastic leukemia. The studies compared a single high-dose influenza vaccine to one standard-dose influenza vaccine.
“Our findings demonstrated that the high-dose influenza vaccine was safe for these populations, and some patients even exhibited higher antibody titers for influenza A antigens,” Halasa said.
These phase 1 studies served as the foundation for the phase 2 project funded by an NIH grant and recently described in NEJM: a nine-center trial involving pediatric stem-cell transplant patients. Participants included a total of 170 patients ranging in age from 3 to 17 years who were between three months and three years’ post-transplant.
The study compared the effects of two doses of the high-dose flu influenza vaccine to two doses of the standard-dose influenza vaccine. It was the first time this inquiry was conducted in a pediatric population, with the team subsequently analyzing the results.
Long-term Goals
“We aimed to gather primary data on this particularly vulnerable group,” Halasa said.
This pediatric population is less likely to respond to the standard influenza dose.
“Given that these children are receiving an entirely new immune system, they are most susceptible to influenza, especially in the initial post-transplant period,” Halasa said.
“Given that these children are receiving an entirely new immune system, they are most susceptible to influenza.”
“In this population, patients are definitely at risk, for severe influenza illness, including death, as well as secondary bacterial infections. The patients in the study tolerated the higher doses well.”
Halasa and her team are continuing their work with additional phase 2 influenza vaccine trials in transplant populations.
“People who are making policy decisions regarding stem cell transplant patients will be able to use this data to help them make vaccine-schedule recommendations,” Halasa said.