Hundreds of women with stress urinary incontinence (SUI) have undergone autologous muscle-derived cell (AMDC) sphincter repair as participants in Cook MyoSite trials.
Ongoing investigations are bringing in larger participant pools to examine efficacy in a broader population, according to Vanderbilt University Medical Center genitourinary medicine leaders Melissa Kaufman, M.D., Ph.D. and Roger Dmochowski, M.D.
The therapy, introduced in 2015, has so far met with largely successful results in improving urinary control in women who have had prior surgery for stress urinary incontinence (SUI).
“This regenerative cell-based therapy is the newest frontier for stress incontinence and the first that offers the potential of a substantial symptomatic benefit, versus simply ameliorating the condition and the symptoms,” said Dmochowski, past president of the American Board of Urology and site principal investigator for the current phase 3 AMDC trial, CELLEBRATE.
“This regenerative cell-based therapy is the newest frontier for stress incontinence.”
“It is also the first regenerative option that’s undergone such a rigorous clinical evaluation. Likely, it will lead the way in providing a variety of regenerative options to the next generation of women who experience this highly prevalent and significantly bothersome condition.”
The AMDC treatments were originally developed with muscular dystrophy in mind. The fact that the cells don’t migrate, as mesenchymal stem cells do, eliminated their candidacy for treatment, but heralded suitability for site-specific uses, including for the sphincter muscles, explained the researchers.
“The results to date indicate that this may be a durable solution over years or decades and, importantly, amenable to re-treatment if needed,” said Kaufman, who serves as global principal investigator on CELLEBRATE.
Limitations of Current Therapy
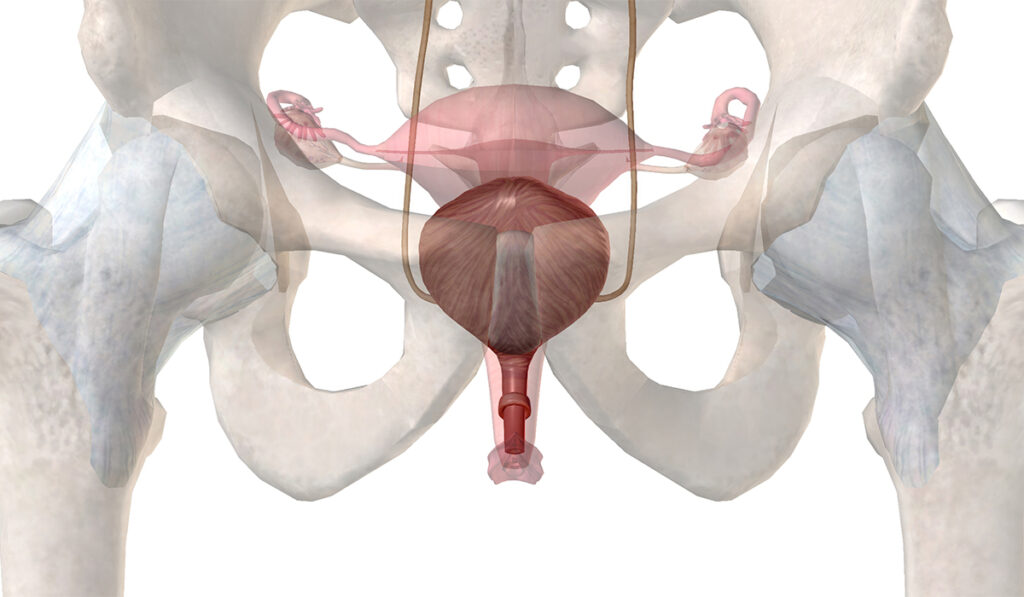
Aging, injury and prior surgeries can degrade the urethral sphincter muscle function, leading to stress incontinence.
“For women, the root of the problem can be hormonal loss with aging, the mechanical distortion that can happen during childbirth, combined with neurologic injury resulting from distortion of the supportive tissues in the pelvis,” Kaufman said.
Current therapies include pelvic floor physical therapy, Kegel exercises, vaginal inserts such as pessaries, injections of urethral bulking agents, and a variety of surgical sling or suspension procedures. But Kaufman says the AMDC urinary sphincter repair is the only procedure that may provide durable and significant sphincter regeneration as opposed to symptom relief.
The process starts by harvesting and culturing approximately 200 mg of the vastus lateralis in the thigh through a minor clinic biopsy procedure. This is injected into the urethral sphincter muscle, where the cells may have several mechanisms of action to regenerate muscle. The injection takes about two minutes and is delivered by a device that allows circumferential delivery around the entire urethra.
Kaufman says patients may see symptom improvement within a few months of the procedure.
Stimulated Progenitor Cells
The cells, a product of terminally differentiated progenitor cells within the skeletal muscle, are stimulated by injury. When grown in a culture and transplanted, they engraft to the sphincter.
“The mechanism is probably threefold: the progenitor cells from the thigh muscle are forming their own myotubes and myofibers; they are also stimulating these progenitor cells in the sphincter to form their own myotubes and myofibers; and they are recruiting new growth factors,” Kaufman said. “The injury from the needle punctures may also stimulate quiescent satellite cells to form muscle.”
Kaufman added that preclinical studies have demonstrated neuroregeneration and angiogenesis take place to support the new muscle matrix.
New Findings
In a second phase 3 AMDC trial, 297 women with SUI were randomly assigned to AMDC urinary sphincter repair or a sham procedure for the one-year study period. Participants who had prior surgery but still experienced recurrent or persistent symptoms experienced a durable 75 percent reduction in symptoms, twice the rate of the placebo group.
“This supports previous suggestions that this population, who have very limited remaining treatment options, may be ideally suited for AMDC urinary sphincter repair therapy,” Kaufman said.
A number of patients without prior surgery who had a greater than a 10 baseline incontinence score also experienced improvement, but there was high variability in their responses, as well as in the placebo response rates across strata, impacting the generalizability of the treatment response.
“Several large randomized clinical trials are pending, and these will be crucial in determining efficacy across all the populations as well as guiding integration of the procedure into clinical practice,” Kaufman said. “Based on experience to date, I think we will find in the future that women who undergo two injections may experience more favorable outcomes.”
Multiple Applications on Deck
At Vanderbilt, researchers have additionally been involved in trials exploring AMDC applications for post-prostatectomy urinary continence. The procedure is also being studied for fecal incontinence, underactive bladder and tongue dysphagia in patients with a history of oropharyngeal cancer.
“This technology will eventually, we sincerely hope, be employed across all these different spectrums and touch so many lives,” Kaufman said.