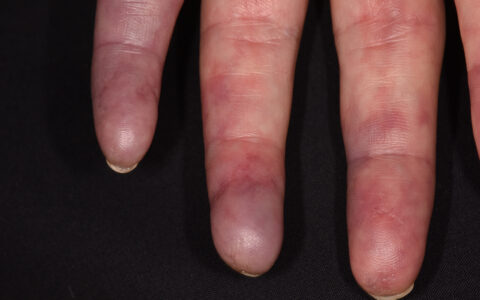
Interstitial lung disease (ILD), arthritis, and the skin condition mechanic’s hand are the classic triad of clinical features common in anti-tRNA synthetase syndrome (ARS). Although some patients with this rare autoimmune disease present with all three, many also present with ILD alone, causing delays in diagnosis.
To address diagnostic delays in a disease that, left untreated, might lead to death within just a few months, rheumatologist Erin Wilfong, M.D., and colleagues at Vanderbilt University Medical Center advocate for the use of comprehensive myositis autoantibody panels.
“We saw a young woman in our intensive care unit with ARS who had nothing other than lung disease – no skin disease, no muscle disease,” Wilfong said. “I usually have a pretty good guess when someone presents with an autoimmune disease, but had we not sent that antibody test, we would have missed this one.”
Issue of False Positives
The American Thoracic Society recommends basic serologic screening in a workup of ILD, but does not specify comprehensive myositis serologies. Wilfong says the issue of false positives may be to blame for the hesitancy, yet basic serologies are prone to miss key markers in patients with ARS and related idiopathic inflammatory myopathies.
“Are false positives a problem? Absolutely. But if a myositis panel is never sent, there’s a good chance these patients with lung-limited ARS won’t see a rheumatologist.”
“Are false positives a problem? Absolutely. But if a myositis panel is never sent, there’s a good chance these patients with lung-limited ARS won’t see a rheumatologist,” Wilfong said.
Research from Wilfong and colleagues confirms that patients with ARS-ILD without prominent extra-pulmonary manifestations are commonly first seen by a pulmonologist and are at high risk of being misdiagnosed with usual interstitial pneumonia/idiopathic pulmonary fibrosis.
Collaborating with Rheumatology
For all these reasons, Wilfong stresses that the benefit of ordering a myositis panel and collaborating with rheumatology is worth the potential undue stress of a true false positive.
“That’s a critical role of the rheumatologist – to recognize the phenotypes and help interpret the labs,” she says.
At Vanderbilt’s rheumatology/pulmonology multidisciplinary clinic, which Wilfong leads alongside Joao de Andrade, M.D., chief medical officer of the Vanderbilt Lung Institute, it’s a priority to see patients with positive myositis panels. Patients positive for the particularly serious anti-MDA5 antibody are seen within roughly 7 to 14 days. The MDA5 autoantibody is associated with rapidly progressive ILD, and death can occur within just three months, Wilfong says.
“We would rather see that patient and say, ‘the CT scan doesn’t really fit with how this can look, we think we’re okay to watch,’ than sit on that antibody and do nothing,” she said.
Targeting Autoreactive B Cells
Knowing that ARS is more precisely characterized by autoantibodies rather than clinical presentation alone, Wilfong and colleagues, including Jennifer Young-Glazer, M.D., an assistant professor in the Division of Rheumatology and Immunology at Vanderbilt, have been working to better understand the most common autoantibody in patients with ARS, Jo-1, and the autoreactive B cells that bind to it.
Not much is known about Jo-1-binding B cells, despite their implication in ARS pathogenesis.
“Now that we’ve developed a method to identify Jo-1-binding B cells in the peripheral blood of patients with ARS, can we develop a way to selectively target them?”
“That’s been one of the goals of our research, to identify Jo-1 binding B cells and characterize them,” Young-Glazer said. “We found they are enriched for CD21low cells, a population linked to other autoreactive B cells in diseases like Sjogren’s syndrome and rheumatoid arthritis, suggesting they might be more pathogenic than we think.”
Their findings may also help inform new therapies for ARS and limit side effects related to rituximab and other existing therapies.
“Now that we’ve developed a method to identify Jo-1-binding B cells in the peripheral blood of patients with ARS, can we develop a way to selectively target them?” Young-Glazer asks. “Rituximab targets general B cells, but the ideal approach would be to more specifically target just this subset.”
The MYSTIC Cohort
Both Young-Glazer and Wilfong stress their research wouldn’t be possible without the Myositis and Scleroderma Treatment and Investigative Center (MYSTIC) cohort and the patients that participate.
Wilfong launched the longitudinal biorepository a few years ago and has recently expanded it to include rheumatoid arthritis and anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis, fueling research into essentially all autoimmune diseases with a propensity to impact the lung. The goal is to use patient samples to better predict disease progression and response to therapy.
Patients can enroll in the MYSTIC clinic from either of Vanderbilt’s rheumatology or pulmonology clinics.