Charcot-Marie-Tooth (CMT) disease is known to cause the peripheral nerves to stop working, resulting in loss of dexterity and the sense of touch in the hands and feet. Over two decades, Vanderbilt University Medical Center researchers have been studying a targeted approach to treat this inherited disease and other neuropathies by looking at rarely examined proteins.
In a new discovery published in the Journal of Biological Chemistry, they have identified the cause of CMT disease, putting researchers on the road to developing therapeutic approaches.
“This was an important step and a highlight for our lab,” said senior author Charles Sanders, Ph.D., associate dean for research and a professor of biochemistry at Vanderbilt. “I am thrilled about the future of this work, translating our data and model cell line work to nervous system cells.”
“I’m excited by the possibility that we could come up with something that may help people with this disease,” said co-author Bruce Carter, Ph.D., a professor of biochemistry and an associate director of the Vanderbilt Brain Institute.
A Discovery Born of Shutdown
The academic shutdown brought on by the COVID-19 pandemic afforded first author Justin Marinko – a graduate student in Sanders’ lab – time to more deeply analyze CMT datasets the team had previously collected.
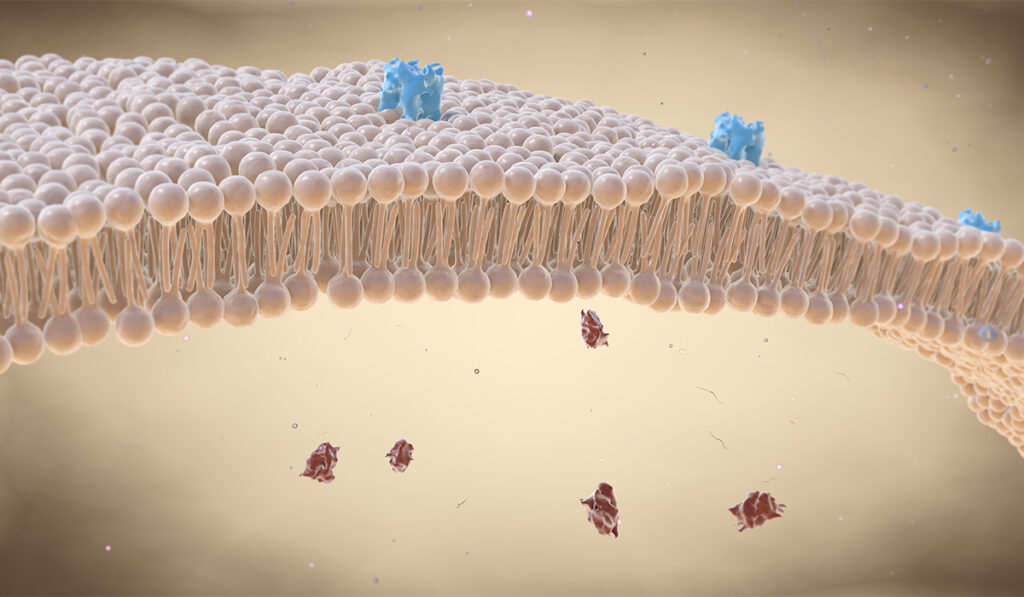
Marinko, who is a recipient of Vanderbilt’s Anne Karpay Award in Structural Biology, began to think about layers within the data that could be analyzed in new ways. Some time to think about the data more thoroughly revealed that overproduction of a CMT membrane protein, PMP22, turns individual cells into traps.
In healthy cells, there are two copies of the gene encoding PMP22 protein, which snakes through the lipid bilayer of the cell several times until it reaches the cell surface. Under disease conditions, a third copy adds more PMP22 to the cell in a way that overloads its pathway to the exterior of the cell — leading to most of the protein getting trapped within the cell and misfolding, where it becomes toxic.
Protein Expression and Impaired Trafficking
Sanders and others have shown that PMP22 trafficking efficiency is related to the stability of its folded structure. The Vanderbilt study explored the relationship between expression levels of PMP22 and its surface trafficking efficiency, and found that there was a direct, negative relationship. Additionally, reduced trafficking efficiency was due more to an increase in internal (likely misfolded) PMP22 at higher total expression levels than to reduced membrane trafficking.
This research is the first experimental evidence that definitively points to this mechanism as the cause of CMT1A, the most common form of CMT. A similar phenomenon likely occurs for proteins in other disorders involving unregulated cell behavior, the researchers said, including some forms of cancer.
“Depending on the conditions, activating or inhibiting PMP22 might help control expression and prevent the disease from progressing.”
The researchers also note long-term accumulation of misfolded PMP22 in Schwann cells and/or the weakened efficiency of protein degradation pathways with aging would help to explain why CMT1A is a progressive disorder.
Translation to Therapeutics
Sanders says there are several directions the team hopes to go with the CMT research. First, they will seek to translate the experiment from cells to living tissue. In addition to PMP22 overproduction, they will explore degradation pathways and the balance between correct forward trafficking of the protein to the cell surface versus accumulation of the protein inside the cell.
A pending NIH grant will help to fund Sanders’ long-term interest in developing therapeutics. “Depending on the conditions, activating or inhibiting PMP22 might help control expression and prevent the disease from progressing,” he said.