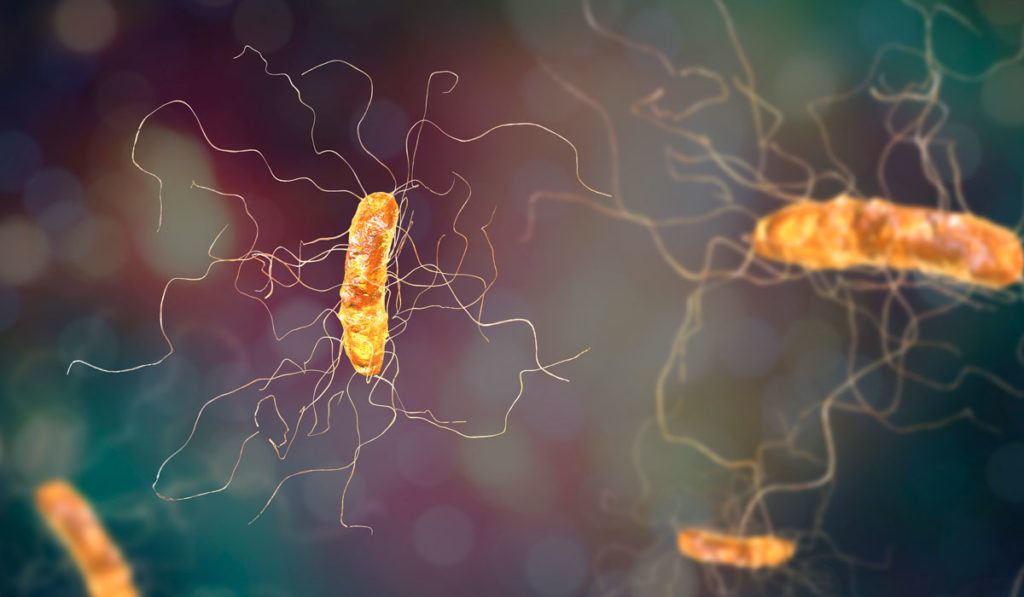
Clostridioides difficile infection (CDI) is increasingly recognized as a public health threat beyond its prevalence as a cause of diarrheal illness in hospitalized adults. According to a 2011 CDC-funded study, CDI has led to 453 million infections and about 29,000 deaths in the U.S. alone. Forty-one percent of these cases were community acquired.
Beyond antibiotics, fecal microbiota transplantation (FMT) is used to treat CDI in both adults and children. “However, in February 2020, an alarming report came out about a small number of immunocompromised adults who contracted antibiotic resistant organisms through FMT, leading to one death,” said Maribeth Nicholson, M.D., an assistant professor of pediatrics at Vanderbilt University Medical Center.
Nicholson was first author on research published in 2019 in Clinical Gastroenterology and Hepatology that established FMT is effective and safe for use in the general pediatric population. More recently, Nicholson says, “We wanted to study its safety and efficacy in immunocompromised children, who are especially prone to CDI.”
Testing FMT in High-risk Children
Nicholson is now co-principal investigator of a large multi-center retrospective trial to assess the use of FMT in pediatric patients with recurrent CDI (rCDI). Initial results are promising.
“We analyzed data from 24 immunocompromised pediatric patients, the biggest cohort of its sort to be studied to date,” Nicholson said. “Eighty-three percent of these patients had no CDI after treatment, which is just a little lower than the results with children who aren’t immunocompromised,” Nicholson said. Four patients (17 percent) experienced CDI recurrence. None of the patients died or had infectious complications related to the treatment.
“We need to focus on this group more,” Nicholson said. “These children were highly hospitalized before the treatment because of CDI, and tended to have many complications. For this cohort, the benefits of FMT seem to outweigh the risk. But a careful discussion of risk and benefit with the families is warranted.”
As another approach, a monoclonal antibody called bezlotoxumab is FDA-approved to prevent rCDI in adults. Nicholson is currently enrolling pediatric patients in a randomized controlled trial to study its safety and efficacy in children, together with C. Buddy Creech, M.D., director of the Vanderbilt Vaccine Research Program.
“For this cohort, the benefits of FMT seem to outweigh the risk. But a careful discussion of risk and benefit with the families is warranted.”
Pandemic Brings New Challenges
SARS-CoV-2 can be transmitted through stool. For this reason, despite the fact that fresh stools generally work better than materials from the frozen stools offered by stool banks, Nicholson says only stools frozen before COVID-19 reached the U.S. are now considered safe, and supplies are dwindling. For many programs, FMT is now on hold.
“We used to use stools donated by a patient’s relatives, which are effective and also free. But with increased screening recommendations to maintain a consistently safe product, many FMT centers, including Vanderbilt, moved exclusively to the use of stool banks. The prior approach spared families having to pay $1,600 to a stool bank for the use of their stool specimens, as insurance coverage is not universal for each course of treatment,” Nicholson said.
Lowering Barriers to Treatment
Without FDA approval, insurance companies do not always cover FMT for CDI. This has led to some families attempting home treatment guided by YouTube videos, Nicholson said. “This is dangerous and not at all recommended.”
“People with recurrent C difficile are desperate to feel better. We need to get universal insurance coverage even though FMT hasn’t gone though the classic FDA drug approval channels.”
“People with recurrent C difficile are desperate to feel better. We need to get universal insurance coverage even though FMT hasn’t gone though the classic FDA drug approval channels,” Nicholson said. She says discussions are underway between patients, clinicians and the FDA to figure out how best to pursue approval and pave the way for coverage, while also providing a standardized and safe product.