It is widely assumed that when confronted with a perceived threat, the cerebral cortex determines how to respond and “filters” its order to the basolateral amygdala (BLA), where the flee-or-freeze response is executed and terminated. But if these fear behaviors persist after the danger lifts, they can become paralyzing and disabling – a key element of post-traumatic stress disorder (PTSD).
To explore how fear becomes entrenched, researchers at Vanderbilt University Medical Center have traveled down the precise neuronal pathways in the brains of mice that trigger fear responses, and which normally extinguish the behaviors once the danger has passed. This scientific journey, detailed recently in Nature Neuroscience, challenges conventional wisdom about how the brain is “remodeled” in response to the intrusion — and subsequent removal — of fear-inducing stimuli.
“You don’t want too much thinking going on” in the face of danger, explained Sachin Patel, M.D., corresponding author on the paper and director of the Division of Addiction Psychiatry at Vanderbilt. “You want very distinct outputs to happen independently so the animal can choose quickly — should I freeze, run or just go about my business?”
Mapping the Fear Response
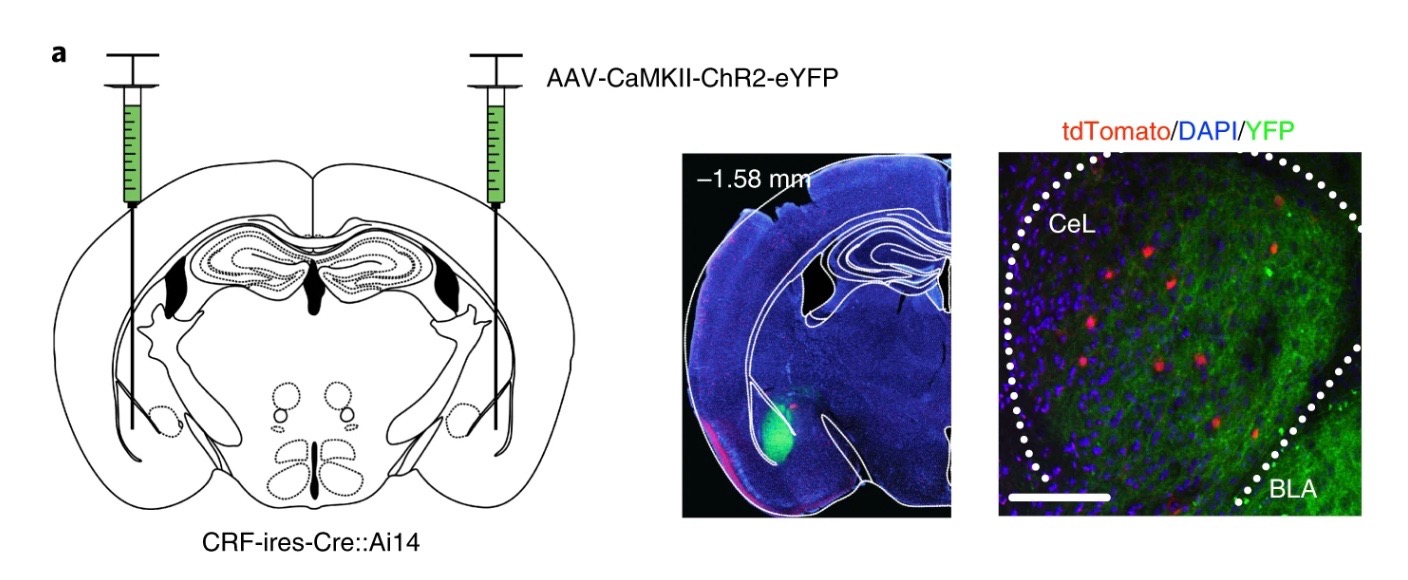
In the study, the researchers discovered something unexpected: acquisition of conditioned freezing behavior is associated with dynamic remodeling of relative excitatory drive from the BLA away from corticotropin-releasing factor-expressing (CRF+) centrolateral amygdala neurons, and toward non-CRF+ (CRF−) and somatostatin-expressing (SOM+) neurons, while fear extinction training remodels this circuit back toward favoring CRF+ neurons. This remodeling is required for optimal memory retrieval.
“A lot of learning-related plasticity and remodeling in the brain is occurring at some of these more ‘primitive’ central amygdala synapses rather than just within the cortical-like areas.”
“A lot of learning-related plasticity and remodeling in the brain is occurring at some of these more ‘primitive’ central amygdala synapses rather than just within the cortical-like areas,” Patel said. “It is here that the animal learns to fear certain stimuli through one neuronal channel and ‘unlearns’ the fear through the other channel once the threat is gone.”
If the association persists after the threat is gone and the environmental cues continue to trigger anxiety and fear, it can lead to PTSD. “It’s like they’re stuck on the freezing channel and can’t flip back to the normal behavior channel,” Patel said. “That’s one theory. Or it might be related to some sort of deficit in this synaptic flexibility mechanism we’ve discovered.”
Implications for Therapy
PTSD is currently treated by gradually exposing patients to the environmental cues that trigger their fear responses in order to help their brains relearn to extinguish the behaviors. But exposure therapy is intense; some patients cannot tolerate the anxiety it can cause.
The next step for the researchers¹ is to look for receptors or proteins expressed by cells in one channel but not in the other. “That would provide opportunities to pharmacologically manipulate the dynamic switching between those channels,” Patel said.
He added, “If we knew that they had a different molecular composition, maybe there’s a way that we could inhibit the ‘freezing’ channel, the fear channel, and promote the other channel.”
Patel said the knowledge gained about the fear response may advance understanding of other brain disorders including drug and alcohol abuse. “Addiction is in part driven by aberrant learning,” he said. “We know the amygdala is important in both fear-learning as well as making associations between the rewarding effects of drugs and environmental cues. A lot of the same mechanisms might be at play.”