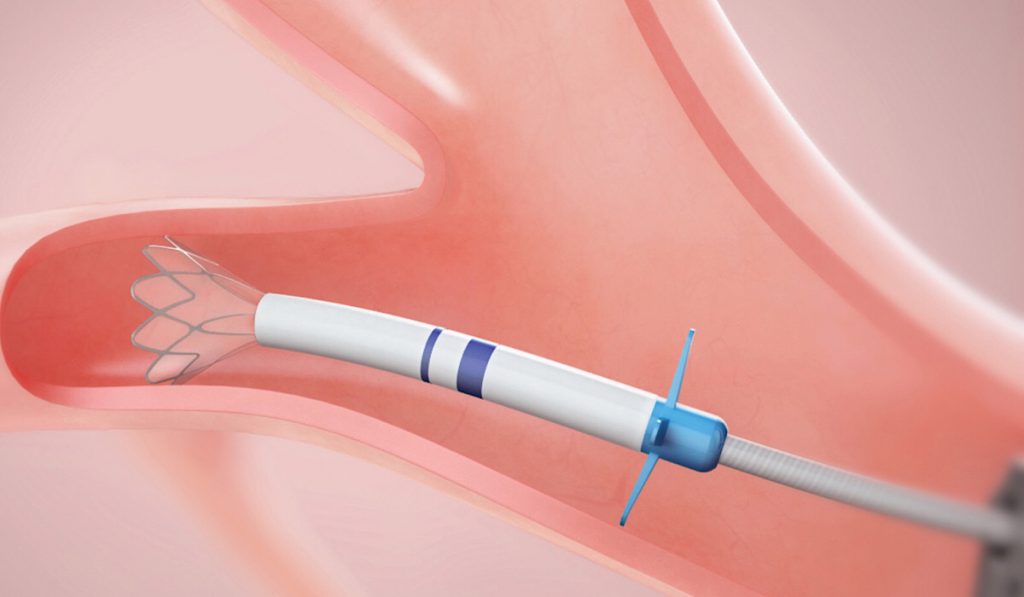
Following early stage medical therapy, treatment options for emphysema are limited to lung volume reduction surgery or transplant. For nearly two decades, scientists have been looking for non-surgical alternatives (e.g. steam or endobronchial coils).
This year, with the completion of the TRANSFORM and LIBERATE trials, two non-surgically implantable endobronchial valve (EBV) devices have been FDA-approved for clinical use in adults. Vanderbilt University Medical Center’s Interventional Pulmonology Program is one of the first centers to receive the Zephyr® valve for implantation.
“This is a big deal for people with emphysema,” said Otis Rickman, D.O., associate professor of medicine and thoracic surgery and founder of Vanderbilt’s Interventional Pulmonology Program. “Previously, patients had to have painful, complex surgery to remove the bad part of the lung and let the good lung reextend. With this new technology, they can be out of the hospital after three days of observation.”
Isolating the Lobe from Airflow
An EBV device is designed to block airways feeding the hyperinflated lobe of the emphysematous lung. When the lobe is isolated from airflow, trapped air escapes only through the valves until the lobe volume is reduced. The remaining lobes are then able to expand more fully and work more efficiently, improving overall lung function.
“In these studies,” Rickman said, “patients have shown improvement in lung volume. They can walk farther and have less shortness of breath. In the trials, people with the valves continued to improve while those not receiving the valves continued to decline.”
EBVs are implanted using a bronchoscope under general anesthesia. The procedure time is typically 30 to 60 minutes, says Rickman. Three to five valves of varying sizes may be placed. Patients must be observed in the hospital for three days post-implantation with a chest tube kit at bedside to watch for pneumothorax. The valves can be removed via bronchoscope if needed.
EBV Patient Profile
EBV candidates are patients with little to no collateral ventilation between the target and adjacent lobes, and a CT scan showing heterogeneous emphysema and complete fissure. The patient cannot be an active smoker, or have an active infection, allergy to nitinol or silicone, or bullae less than 30 percent. Indications against EBV include prior lung transplant or reduction surgery, median sternotomy, lobectomy, or coexistent disease such as bronchiectasis or severe fibrosis.
Vanderbilt is currently evaluating patients for Zephyr® implantation. Patients under consideration will first receive pulmonary function testing and a CT scan analyzed with advanced software to determine if the distribution of emphysema meets criteria for device implantation. After this initial screen and more extensive testing, followed by six weeks of optimal medical therapy including inhalers and rehabilitation, the procedure can be performed.
“This is the first procedural treatment for emphysema that is non-surgical,” Rickman said. “Patients can stay on their current meds and hopefully, will not have to progress to more invasive interventions.”
Rickman and his team of interventional pulmonologists offer other advanced diagnostic and therapeutic procedures including rigid bronchoscopy, airway stents, navigational bronchoscopy, endobronchial ultrasound, medical pleuroscopy and low-dose CT scanning for lung cancer.