It has long been known that acute kidney injury (AKI), even after it resolves, can set the stage for chronic kidney injury. What has been poorly understood is the process by which this occurs and how to prevent it. Now, a study forthcoming in Science Translational Medicine illuminates the chain of molecular events that trigger fibrotic secretions and render the kidney increasingly at-risk for failure.
“This collaboration has generated the first body of research to get at the mechanism for how molecular changes in the kidney cells lead to fibrosis.”
The leading causes of AKI are ischemic reperfusion injury, low blood volume from dehydration, an immune response to sepsis, and nephrotoxins. Following AKI, fibrotic factors are oversecreted, depositing collagenous scar tissue around and in place of healthy cells. Worse, this process triggers a proliferation of more fibrotic tissues over time, often eluding notice until the kidney damage reaches a critical mass of around 80 percent, when creatinine levels finally exceed the normal range.
To help elucidate how kidney fibrosis develops, Vanderbilt cell biologist Craig Brooks, Ph.D., collaborated with investigators at Harvard Medical School and Brigham and Women’s Hospital as well as colleagues in France and Japan. The group has conducted multiple studies in tandem. “This collaboration has generated the first body of research to get at the mechanism for how molecular changes in the kidney cells lead to fibrosis,” Brooks said.
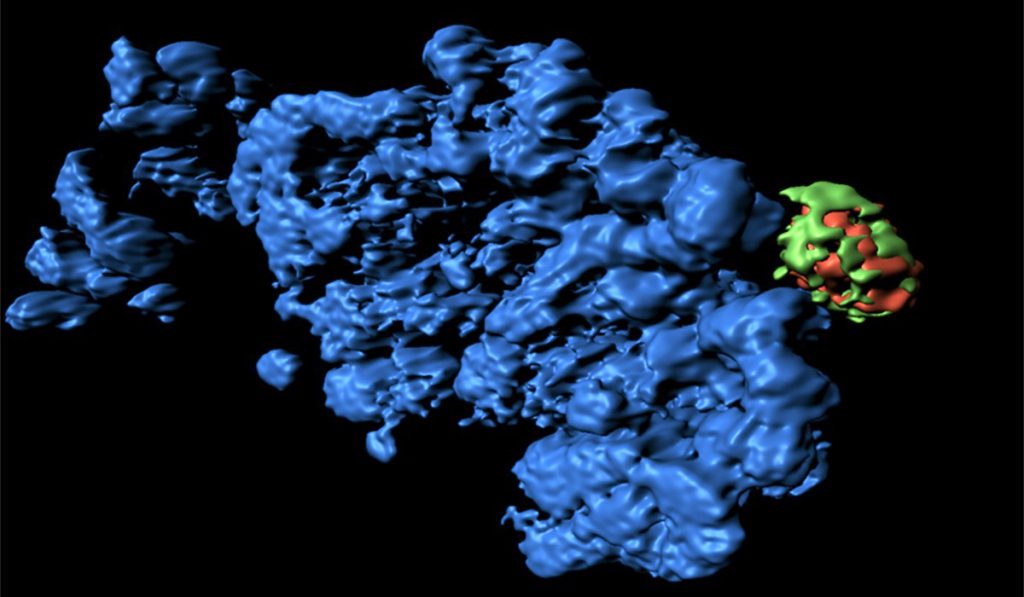
Brooks and colleagues used mouse models to analyze the profibrotic cascade in proximal tubule cells (PTCs). They also conducted conformational work with human tissue samples.
The Pathway to Fibrosis Revealed
The researchers began with the prior knowledge that cell cycle arrest in the transition between the G2 and M phases (a control checkpoint where the cell’s fitness to begin mitosis is determined) is complicit in generating fibrosis. “Until now we have seen this coexistence, but there has been a black box around that connection,” Brooks said. “Now we’re connecting the dots.” This required finding the trigger for G2-M phase arrest.
The G2-M phase is a control checkpoint where the cell’s fitness to begin mitosis is determined.
G2-M arrest can fulfill an important protective role over the short term, keeping cells with AKI-induced DNA damage from reproducing. Most cells in G2-M phase arrest either try to fix their DNA and start dividing again, or they die. The problem lies with a small subset of these cells which stay in the G2-M arrest phase for an extended period, secreting profibrotic factors as senescent cells do.
They found that the culprit in G2-M arrest is a normally inactive gene, Cyclin G1, which becomes upregulated in the PTCs of mice shortly after AKI. Because Cyclin G1 is only expressed after AKI, it points to a causal relationship with G2-M arrest.
With this relationship established, the next dots to connect were between the damaged cells that go rogue following G2-M arrest and the downstream deposition of fibrotic tissues, something that begins weeks after the kidney injury.
The researchers analyzed the PTCs at 7-42 days after AKI and found cellular compartments known as the TASCC complex coexistent with the extended G2-M phase arrest. The presence of this TASCC complex is characteristic of senescent cells, regulating the secretion of cytokines and other factors that can drive different cell signaling processes, including fibrosis. “We found that by creating a blockade against TASCC formation, we could prevent the cells from secreting profibrotic factors,” Brooks said.
“Fibrosis is the Final Common Pathway for all Chronic Diseases”
“Our research revealed at least two targets for therapies, maybe more,“ Brooks said. Exploring ways to downregulate Cyclin G1 expression—the trigger—may prove particularly auspicious. Because Cyclin G1 is not expressed anywhere but in the injured cells, regulating it would not impact other cells, thereby averting adverse side effects. Other therapies can take aim at blocking the TASCC complex downstream, where the secretion of profibrotic factors actually occurs.
Brooks and colleagues plan to dig further into the molecular pathway, studying the role of Cyclin G1 in different models and different forms of kidney injury. “Our research has opened the door for us to break down every step in the pathway and determine the best place for therapeutic intervention,” Brooks said. “We are also developing organoids [mini-kidneys grown from human stem cells] to move our research closer to relevance in a human setting.”
The implications are far-reaching. “We have identified the G2-M phase profibrotic mechanism in other organs like the liver,” Brooks said. “And we see similar Cyclin G1 upregulation and TASCC processes in the liver as well.”
“There is a famous line a lot of people use when talking about fibrosis: ‘Fibrosis is the final common pathway for all chronic diseases.’ This is true whether it’s heart disease, lungs, liver or kidney. We hope these findings will have therapeutic implications for multiple organ systems,” Brooks said.