The human body is very efficient at promoting hydroxyapatite crystal formation in bone where it is essential for biomechanical strength. Under healthy conditions, the body is equally efficient at preventing hydroxyapatite crystals from forming in soft tissues where they would interfere with muscle and organ function. It is a mystery why in some conditions, such as severe injury, aging and diabetes, the body is less efficient at maintaining hydroxyapatite crystals in bone, yet plagued with hydroxyapatite crystal formation in soft tissue.
For example, bone diseases, such as osteoporosis or fracture non-union that result in loss of hydroxyapatite where it is needed, often coincide with an increase in hydroxyapatite formation in soft tissue, such as atherosclerosis or heterotopic ossification.
The Paradox of Pharmaceutically Treating Calcification Problems in Patients
This paradox presents a pharmacologic roadblock in treating patients with calcification problems. For example, administering calcium-vitamin D supplementation to women to prevent osteoporosis, while effective, may have the unwanted side effect of driving coronary artery disease. Or, following severe trauma, an anti-bone morphogenic protein (BMP) pharmaceutical may effectively prevent heterotopic ossification in muscle compartments, but it runs the risk of preventing fracture repair.
A Unified Theory of Calcification Disease
These observations led the Schoenecker Lab of Musculoskeletal Tissue Injury at Vanderbilt University Medical Center to hypothesize that there are essential systemic regulators of hydroxyapatite formation whose functions are anatomically specific. In other words, these factors promote hydroxyapatite formation in bone compartments and simultaneously prevent it in soft tissue compartments. If so, a loss of one such factor would explain the seemingly paradoxical conditions described above and provide a singular therapy to restore the balance of hydroxyapatite formation.
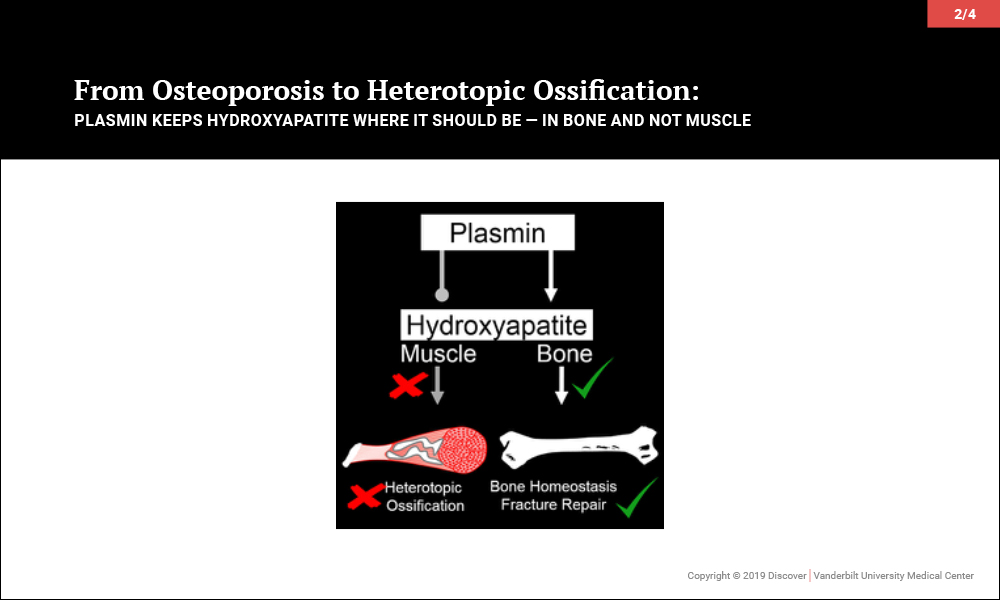
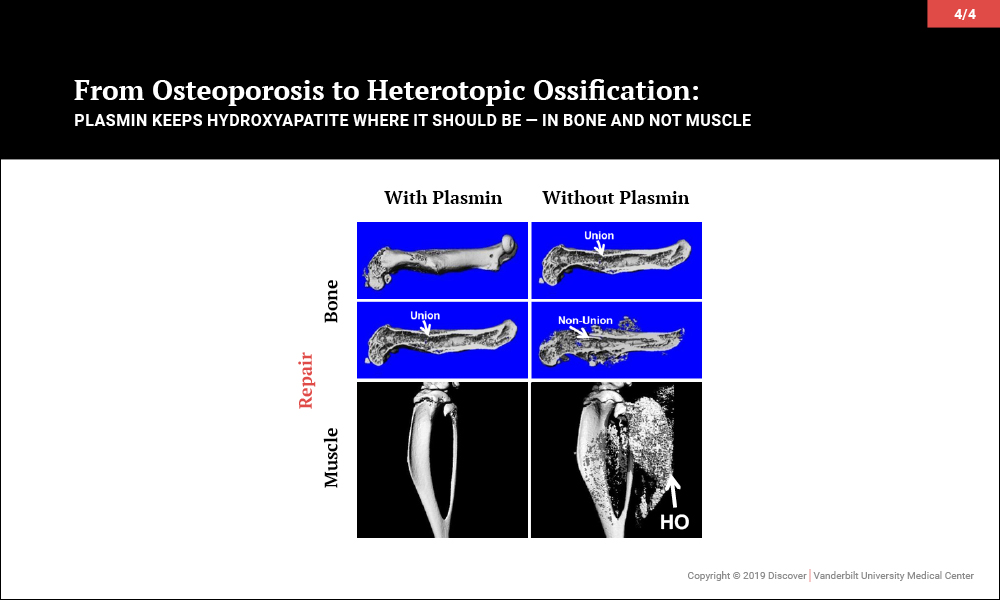
Plasmin Promotes Calcification in Bone and Prevents It in Muscle
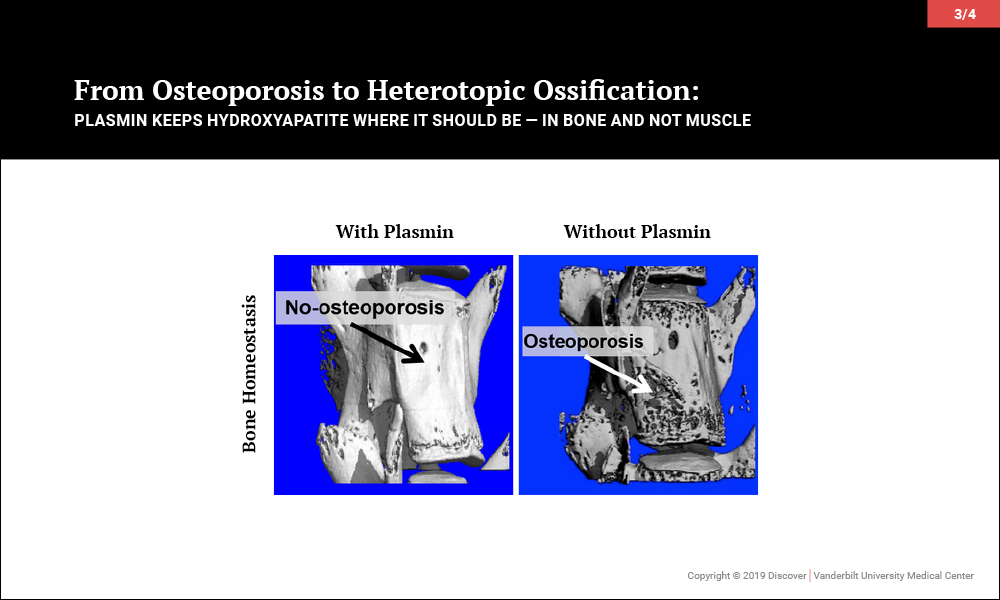
Surprisingly, investigations led to the discovery that plasmin, the primary protease of the fibrinolytic system, may be key to maintaining the balance of promoting bone biology while averting soft tissue calcification problems. In a series of papers, the team of orthopaedic-pharmacologists at Vanderbilt reported that plasmin is essential in preventing osteoporosis, for fracture repair without heterotopic ossification and, most recently, to prevent heterotopic ossification following an isolated muscle injury.
The Potential All-in-One Therapy for Calcification Disease
“An ideal treatment would prevent calcification in soft tissue and also be good for bone biology.”
Given that plasmin activity is known to be impaired in aging, severe injury and diabetes, among other diseases, these findings suggest that future therapies increasing plasmin activity may effectively prevent soft tissue calcification without adversely affecting systemic bone physiology or concurrent muscle and bone regeneration.
“An ideal treatment would prevent calcification in soft tissue and also be good for bone biology,” said Jonathan Schoenecker, M.D. the Jeffrey Mast Associate Professor of Orthopaedics at Vanderbilt. “That’s what we have discovered. Plasmin prevents calcification in muscle, and it is also essential for fracture healing while protecting against diseases such as osteoporosis.”
Future Trials
In its most recent published study, the Vanderbilt group teamed up with Ionis Pharmaceuticals and focused on use of the genetic therapy antisense oligonucleotides (ASOs) to remove the plasmin inhibitor 2-antiplasmin to enhance plasmin’s activity following a muscle injury. The ASO therapy not only prevented soft tissue calcification but also greatly enhanced muscle repair. Vanderbilt researchers are now progressing toward clinical studies with the ASO therapy in settings where plasmin activity is known to be deficient, including severe injuries such as Level-1 motor vehicle accidents and burn injuries.
In addition, the Vanderbilt team has partnered with Pseudoxanthoma Elasticum International to determine if plasmin therapy is sufficient to reduce and prevent hydroxyapatite formation in skin, eyes and blood vessels. This partnership may lead to clinical studies aimed at improving tissue recovery and preventing calcification in patients susceptible to diseases of dysregulated hydroxyapatite formation.
The most recent Vanderbilt research was published in the August 2014 issue of Arthritis and Rheumatology (vol. 66, no. 8), August 2015 issue of The Journal of Clinical Investigation (vol. 125, no. 8), February 2017 issue of the Journal of Bone and Mineral Research (vol. 32, no. 2) and reviewed in the October 2015 issue of The New England Journal of Medicine.
These projects were led by Masato Yuasa (M.D., Ph.D.), Stephanie Moore (B.S.), Heather Cole (M.D.), Nicholas Mignemi (Ph.D.), Courtney Baker (M.D.), Matthew Flick (Ph.D.), Justin Cates (M.D., PhD.), Herbert Schwartz (M.D.) and Jeffry Nyman (Ph.D.). The study was supported by The Fighting Duchenne Foundation, The Orthopaedic Trauma Association, The Orthopaedic Research and Education Foundation, Pediatric Orthopaedic Society of North America, the National Institutes of Health (grants HL007751, GM007628, AR065762, RR027631, DK007061), the Vanderbilt Institute for Clinical and Translational Research, the Howard Hughes Medical Institute, the Vanderbilt Orthopaedic Institute and the Caitlin Lovejoy Fund.