Multiple myeloma follows non-Hodgkin lymphoma as the second most common blood cancer, and one for which there is no curative therapy. While a battery of drugs have been developed to treat the disease, side effects have been a secondary consideration, given the great need to keep the cancer in check.
Now, researchers are quantifying what has been a clinical awareness that carfilzomib can have serious cardiovascular (CV) side effects. A study published in the Journal of Clinical Oncology found CV events in over half of patients treated with the drug. Robert F. Cornell, M.D., clinical director of Plasma Cell Disorders at Vanderbilt-Ingram Cancer Center, was primary investigator on the study.
“Our evidence justifies a more circumspect approach to therapy, involving close monitoring of high-risk patients and modulation of preventative cardiovascular health measures.”
“Carfilzomib is a potent and very effective chemotherapy for our patients. However, our study found more CV events than studies previously recognized,” Cornell said. “While we need verification of these findings, our evidence justifies a more circumspect approach to therapy, involving close monitoring of high-risk patients and modulation of preventative cardiovascular health measures.”
Key Allies in the Fight
Irreversible proteasome inhibitors, including carfilzomib and bortezomib, form the backbone of anti-myeloma therapy. The new study looked at CV events associated with both drugs.
Carfilzomib’s use since 2012 has contributed in large measure to a life expectancy extension, with some multiple myeloma patients now surviving over a decade, Cornell said. Carfilzomib works by targeting the mechanism by which proteins within the cancer cells are deployed to support new cell growth. By preventing the release of these proteins, carfilzomib causes them to build up in the cell, causing apoptosis.
Risks Exceed Expectations
The study enrolled 95 patients, with 65 taking carfilzomib and 30 taking bortezomib. Cardiovascular events occurred in 51 percent of the carfilzomib cohort and 17 percent of the bortezomib cohort. Events included heart failure, hypertension, arrhythmia, acute coronary syndrome and one case of sudden cardiac death. Eighty-six percent of the cardiovascular events occurred in the first three months of treatment.
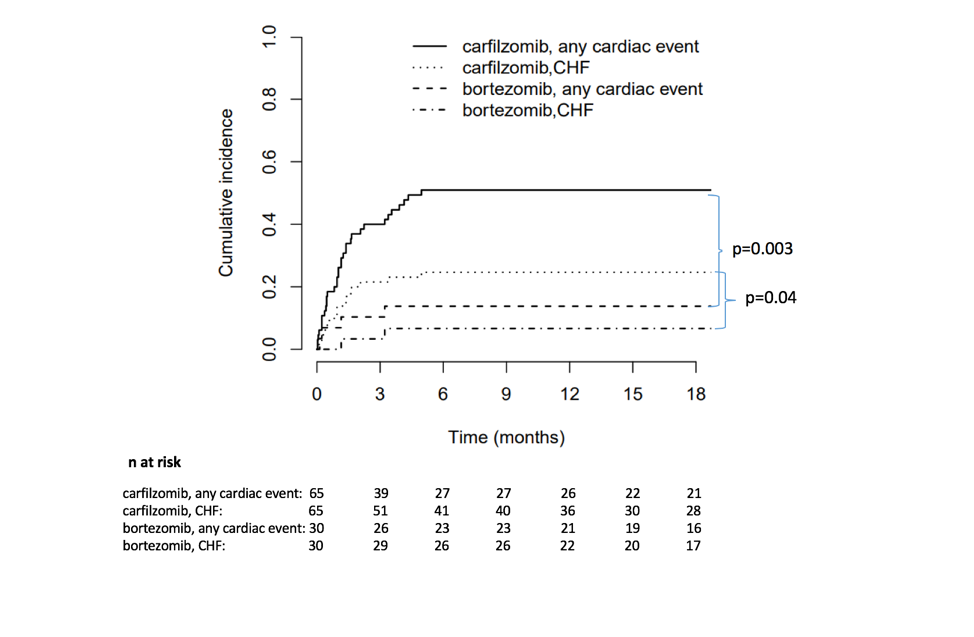
“The number of CV events was higher than what was reported in clinical trials, which tend to include younger, healthier patients with fewer comorbidities,” Cornell said.
Keeping Patients Safe
Cornell recommends monitoring patients’ natriuretic peptides (BNP and NTpro-BNP), which are highly predictive for CV events, at day one and day 15 of each chemotherapy cycle for at least the first several cycles of treatment. “If levels become elevated, closer monitoring support of care changes should be considered.”
These may include dose delays, dose reductions, a slower infusion rate, or in the case of heart failure threat, diuretics or IV fluid quantity modifications. “Patients with elevated natriuretic peptides and a medical history of cardiovascular disease are at greatest risk for CV events and should be seen by a cardiologist prior to and during the therapy. That way, problems can be acted upon quickly, with the goal of preventing dose reductions or dose delays with this important treatment,” he said.
Unlocking the Mechanism
Cornell and fellow researchers are now working to understand mechanistically why these CV events occur, and to identify ways to target malignant cells and spare healthy cells.
“Everyone is hopeful cellular therapies will be the next phase in anti-multiple myeloma therapy.” Some of the most promising therapies involve modulating patient’s own immune system to fight the myeloma cells, using biospecific antibodies and CAR-T cell therapies which specifically target T cells.”
Until then, combinations of chemotherapy, maintenance therapy and stem cell transplant will constitute the arsenal against this still incurable disease.