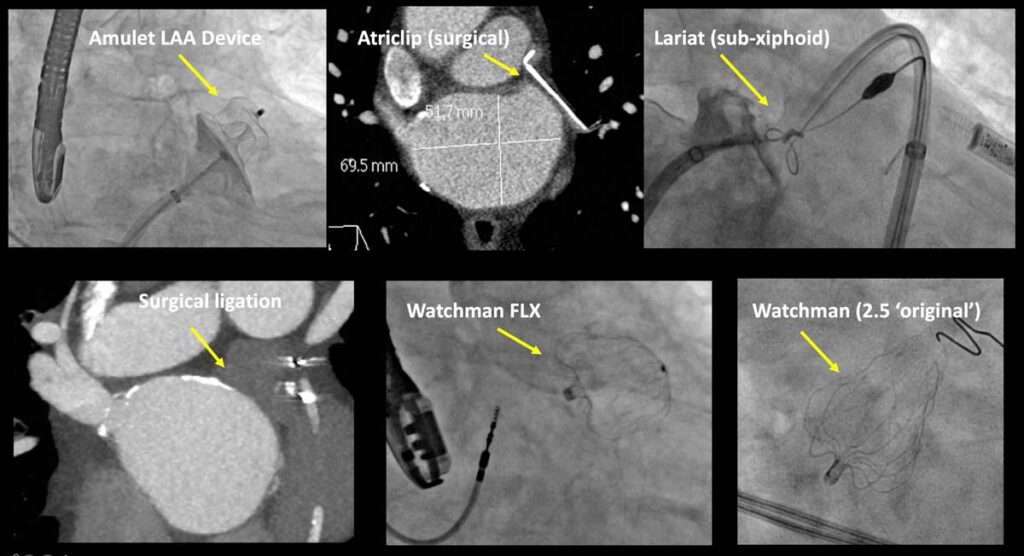
Christopher Ellis, M.D., director of the Left Atrial Appendage Closure Program at Vanderbilt University Medical Center, has been intimately involved in testing catheter-based occluder devices, which evolved from the original Watchman 2.5™ to the Watchman FLX™. Recently, much of his focus has been on the latest left atrial appendage (LAA) device to market – the Amplatzer™ Amulet™.
Ellis was an author on the recent Circulation publication that compared the devices. Results of the four-year study led to FDA approval for the Amplatzer Amulet occluder in August 2021. “We found the Amulet to be noninferior to the Watchman device for the primary safety endpoint, and superior for LAA occlusion,” Ellis said. “There were slightly higher procedure-related complications with the Amulet, but these decreased with operator experience.”
“I think we needed to have device alternatives on the market, and the Amulet has proven itself,” he added. “Now there are not only better drug options, but there are minimally invasive procedures, and we are testing all of these – the lariat procedure and the Amulet, Watchman 2.5 and Watchman FLX devices – as part of our current research.”
Candidates and Confounders
About 95 percent of embolic clots originate in the LAA. An LAA closure device is indicated for patients at increased stroke risk due to non-valvular atrial fibrillation (NVAF), particularly if they have significant bleeding risk from anticoagulants.
“I think we needed to have device alternatives on the market, and the Amulet has proven itself.”
Device placement may be made more difficult by patient anatomy. “Larger LAA necks and those with irregularly shaped perimeters (elliptical opening, complex LAA lobes) make for more challenges in assuring a tight seal with no risk of dislodging or embolizing,” Ellis said. “Still, only about two to three percent of people we screen fall outside the bell-shaped curve of what we can currently close.”
The procedures themselves have a very low complication rate, Ellis says, but the major concern with implants is that peridevice leaks can develop at any point. Ellis and colleagues recently analyzed delayed leaks after Watchman placement. “We have found that leaks tend to get bigger over time,” Ellis said. “Even when the leak is just two to four millimeters in size, there is an associated increase in stroke risk.”
Elllis helped test the Watchman FLX alone in the PINNACLE-FLX trial, but this device has not been directly compared to the Amplatzer Amulet. Ellis says the more flexible design of Watchman FLX makes it a better closure device for most LAA anatomies than the original device. “But we still want to get to the point where there are zero leaks around these devices,” he said.
Similar Safety and Effectiveness
In the Circulation study, Ellis and colleagues randomly assigned 1,878 study participants with NVAF to either the Amplatzer Amulet or the Watchman 2.5 (the version available at the time). The primary endpoints were safety, which included procedure-related complications, all-cause death or major bleeding at 12 months, and effectiveness – a composite of ischemic stroke or systemic embolism at 18 months and the rate of LAA occlusion at 45 days.
The devices demonstrated similar profiles, with 97 percent of participants avoiding stroke during the 18 months following implantation, in the absence of anticoagulant therapy. “These were patients with an average CHADS 2 Vasc score of 4.5, which would predict a stroke rate of about 8 percent over 18 months, off anticoagulation. This means we saw over a 70 percent greater reduction in the risk of stroke, about the same as what Eliquis® does,” Ellis said.
Ellis says he and others are continuing to monitor the outcomes longitudinally. “The dual-seal technology of the Amulet gives it an extra lip – much like a manhole cover design – that is responsible for the better occlusion. How much of a difference that ultimately makes is still unknown.”
The Declining Role of Anticoagulants
Now, LAA occluder devices are going head-to-head with anticoagulants like Eliquis, Xarelto® and Pradaxa®. Ellis says about three percent of patients on anticoagulants have major bleeding issues each year. “With the gap between safety profiles of anticoagulants and devices fast closing, the devices are becoming the treatment of choice for those at increased risk for bleeding,” Ellis said.
Vanderbilt is now a study site for trials comparing medical management to the Amplatzer Amulet (CATALYST trial) or Watchman FLX (CHAMPION-AF trial) devices. “For some longstanding patients, you may now be asking the question, ‘Is it potentially better to close the LAA than even use blood thinners at all?’.” Ultimately, Ellis expects that a combination of mechanical closure and low-dose anticoagulants may become a common means of further improving safety for NVAF patients.