New research out of Vanderbilt University Medical Center offers a paradigm for how chronic inflammation alters vasomotor function during cardiovascular disease.
The work, led by pediatric cardiologist Fred Lamb, M.D., Ph.D., centers around how certain anion channels seated in smooth muscle cell membranes regulate the impact of high levels of reactive oxygen species generated during inflammation. These LRRC8 channels are thereby able to modulate vascular reactivity.
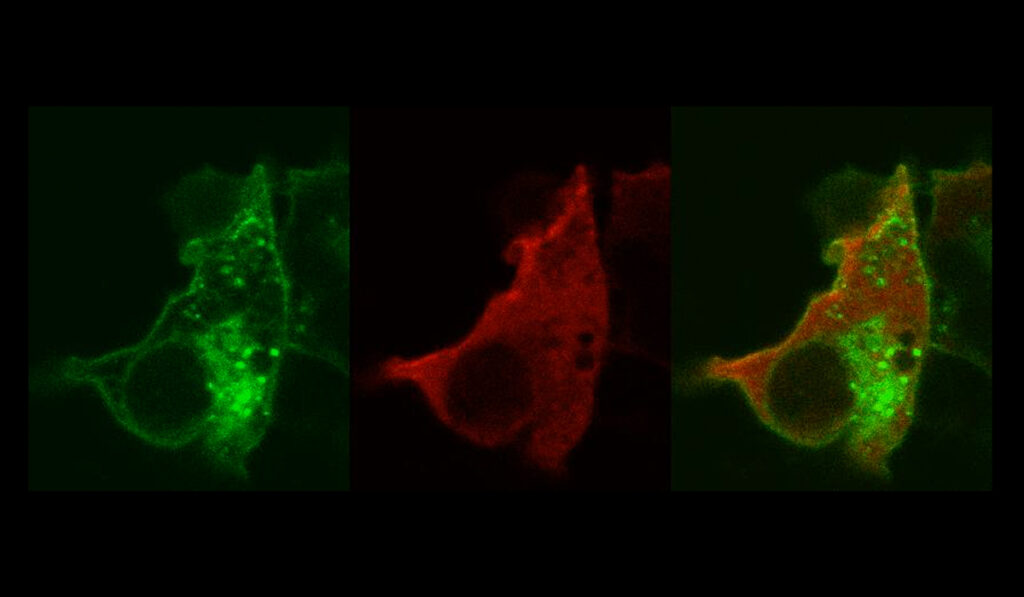
In their new study, Lamb’s team outlined this happens by identifying a binding partner for the channels, myosin phosphatase Rho interacting protein, or MPRIP for short. The results were published in The FASEB Journal.
“MPRIP is a scaffolding protein that brings other things to the party,” Lamb said. “It brings in actin and myosin and other proteins like RhoA that control contractility, and it turns out all of that is oxidant-sensitive machinery.”
Beyond Antioxidants
Results from the study also help explain how oxidants normally function in vascular smooth muscle.
“This gives structure to the system so people can ask more refined questions,” Lamb said.
Reactive oxygen species are essential messengers in the body. They help signal cells to modulate vasodilation or vasoconstriction. But when levels get too high, such as during chronic inflammation, oxidants can have damaging effects.
“We’ve been throwing antioxidants at things for 30 years, and it hasn’t really worked,” Lamb explained. “Part of that is because oxidants are really important to run normal reactions. And when you give a massive dose of vitamin C or E, you may be stopping some off-target oxidation, but you’re also interfering with normal redox signaling.
“Figuring out how to more specifically interfere with pathologic redox signaling is what we’re going after here.”
Detailed Dive
Lamb and colleagues describe a series of binding partners that are activated by oxidants as they enter vascular smooth muscle cells via LRRC8 anion channels.
The work complements cell-based studies by Lamb and colleagues that identify Nox1 as a key intermediary between LRRC8 channels and oxidant production. Nox1 helps produce superoxide, which then flows into cells via the channel, where it interacts with specific proteins, among them MPRIP.
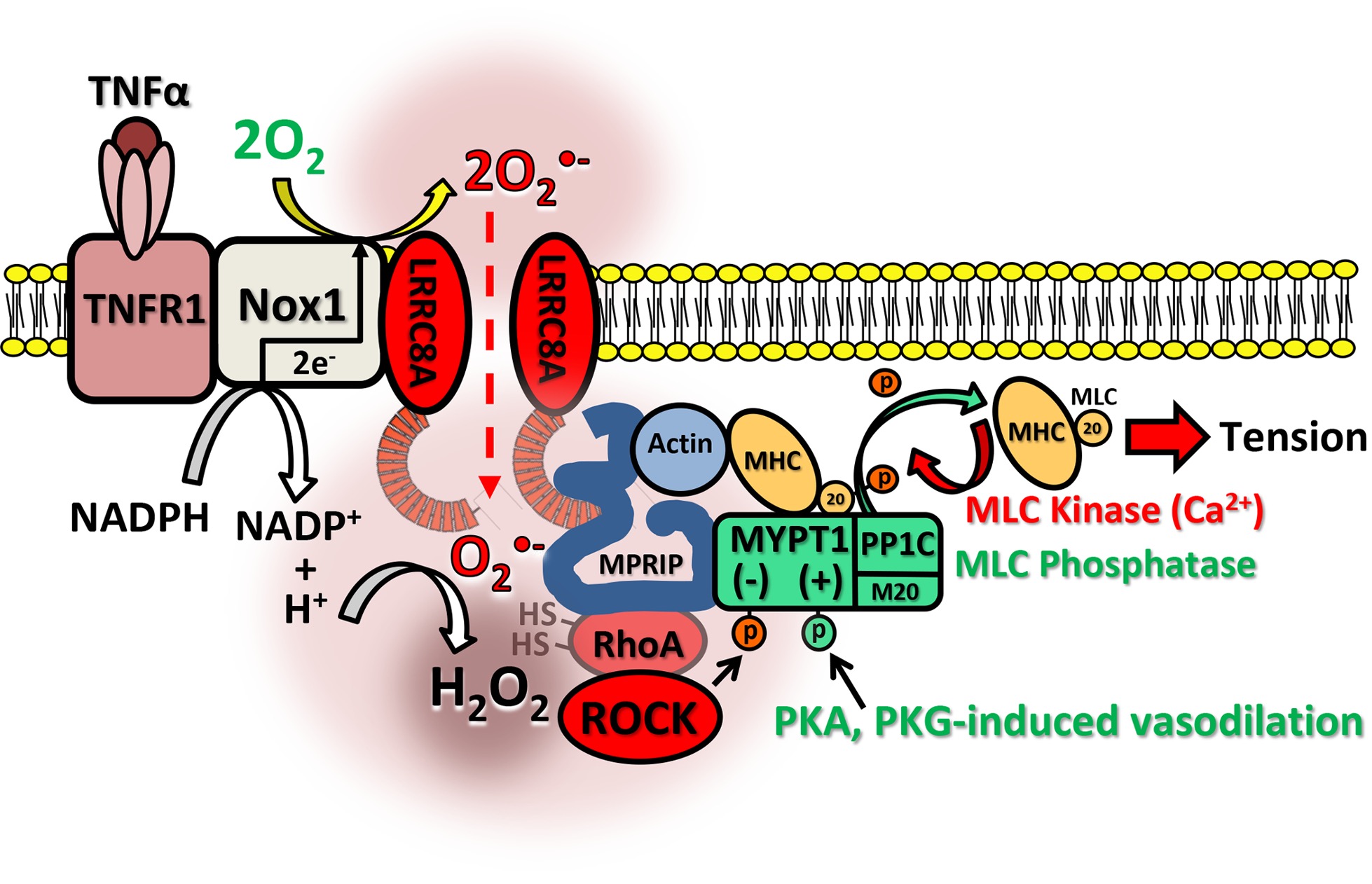
LRRC8 channels have five subtypes, labeled A through E. Lamb’s team focused on 8A, which is embedded in every channel, to create a knockout mouse model. Mice in the study were devoid of LRRC8A channels in smooth muscle cells responsible for vasomotor function.
The genetically modified mice enabled the researchers to study oxidant signaling downstream of the channel in a whole animal for the first time.
“We saw not only a reduction in inflammation, but also an augmentation of vasodilation, and impairment of constriction,” Lamb said.
Murine arteries lacking the channel appeared resistant to inflammation even after two days of ex vivo exposure to TNF-alpha.
“In fact, they functioned better,” Lamb said.
The lack of LRRC8A channels made the knockout mice essentially resistant to vascular inflammation.
“In these mice, we saw not only a reduction in inflammation, but also an augmentation of vasodilation.”
Toward Therapeutics
The mice demonstrated the importance of LRRC8 anion channels to the cardiovascular phenotypes Lamb sees every day in the hospital. And, how modulating channel function might be therapeutic.
“Targeted cytoplasmic delivery of reactive oxygen species can alter vasomotor function and contribute to vascular disease,” the researchers wrote. “Anion channel inhibition may provide a novel approach to treatment of vascular inflammation.”
Lamb envisions a way that LRRC8 channel inhibitors, like montelukast or tamoxifen, might be repurposed to address vascular inflammation.
“There are already clinically approved drugs out there that block this channel,” Lamb said. “For example, there are protocols for high-dose Singulair for people with asthma to reduce hypercontractility. Now we may understand a bit more about why that works.”
The researchers are interested in determining whether modulating the channels may have broad utility, given that excessive vascular inflammation and oxidant production is associated with several conditions.
“The next step is to test this in other scenarios, such as sepsis, and to breed the knockout mice with disease models, such as mice with induced hypertension, atherosclerosis, or diabetes, and see if there is still protection. We’re working on that.”