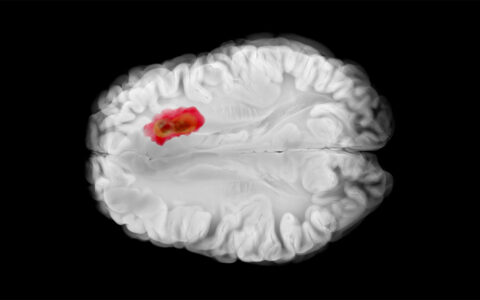
An MRI-guided focused ultrasound system that noninvasively modulates areas of the brain tied to pain and movement has been developed at Vanderbilt University Medical Center, a potentially revolutionary advance in treating neurological and psychiatric conditions.
The new system answers the call for a treatment that is both noninvasive and able to access the deep brain, a combination not available to patients through existing neuromodulation modalities.
The integrated system was developed by VUMC biomedical engineers Charles Caskey, Ph.D., William Grissom, Ph.D., and radiology researcher Li Min Chen, M.D., Ph.D. Co-investigators Dario Englot, M.D., director of functional neurosurgery at Vanderbilt, and John Gore, Ph.D., director of the Vanderbilt Institute of Imaging Science (VUIIS), are engaged in its testing.
The focused ultrasound system is the first to enable visualization of low-frequency ultrasound waves capable of stimulating the deep brain alongside brain-activity imaging using functional MRI (fMRI). Prior to this, deep-brain research relied on simulations derived from a fixed CT scan, whereas the new system allows for real-time imaging of the modulating ultrasound and brain activity.
“The visibility, mapping and feedback enabled by our device allows us to establish targets, direct beams along the planned pathway, and actually see the targets light up.”
“There are other laboratories doing great work developing low-frequency ultrasound to try to affect conditions like obsessive-compulsive disorder, schizophrenia, epilepsy, but without excellent MRI integration, other systems cannot see their targets and their beams,” Caskey said.
“The visibility, mapping and feedback enabled by our device allows us to establish targets, direct beams along the planned pathway, and actually see the targets light up as blood oxygen level dependent signals change when they are reached.”
“This technology is safe and has had a proven impact in animals,” said Chen.
Caskey’s and Chen’s preclinical research formed the foundation for a $4.6 million federal grant to study the technology in humans.
“Besides its potential clinical benefits, it also opens the door to broad research on brain circuitry to help us address myriad pathologies.”
How It Works
With the support of VUIIS, Caskey, Grissom and their colleagues created a custom transducer that integrates into fMRI to enable real-time mapping of ultrasound waves and brain activity. The ultrasound energy used for this fMRI-guided process is higher than for clinical imaging, but lower than high-intensity focused ultrasound (HIFU). It penetrates the skull and modulates the brain but does not ablate tissue, the researchers said.
A magnetic coil integrated with the ultrasound transducers is placed around the patient’s head. The system then transmits multiple ultrasound beams into the brain, much in the way radiation waves are transmitted during stereotactic radiation to converge on a tumor site. The fMRI coil registers the ultrasound waves magnetically, providing a map of the beams and targets.
Chen explains that on the micro level, the ultrasound evokes temporary compression and displacement of cells along the beam paths. When the beams converge on the neural circuit target, they deform the neuronal cell membranes, affecting ion channel activity. This may either stimulate or suppress action potential in the neurons.
“We have known since the turn of the century that ultrasound can modulate the behavior of neurons,” Caskey added. “But this knowledge was not very useful until now. With guided fMRI, we can set the targets, then direct and adjust the beam paths and dosing in real-time, based on system feedback, to reach the cells in the desired regions.”
Two Grant Goals
The grant to further develop the technology and test this system was awarded by the National Institute of Neurological Disorders and Stroke through an initiative called HEAL (Helping to End Addiction Long-term). The team’s primary interest is in neuromodulation of pain perception in post-stroke patients, many of whom experience nerve damage and severe neuropathic pain. The hope is that the system could be a powerful tool in avoiding opioid and other addiction in this population – and beyond.
First, however, the team will test the system in treatment of essential tremor.
“This is a logical first step, since we know that essential tremor can be relieved by HIFU ablation, and motor outcomes are quicker and easier to measure than pain perception,” Chen said. “Can we modulate this condition without ablation? Our preclinical work indicates we can.”
By 2025, the researchers hope to move to the stroke-related pain trials.
Transition to Practice
Chen has spent many years working with animals and humans to try to understand the particular neural circuits involved in pain.
“With post-stroke pain, as well as other kinds of pain, when patients experience a transition from acute to chronic pain, their whole network changes, shifting from more engagement of sensorimotor networks to involving more cognitive, affective, and emotional networks and drastically different patterns of activity,” Chen said.
This shift may help explain the depression associated with chronic pain, Chen said.
She says the initial study machine used 7 Tesla MRI, but the system is now built on 3 Tesla MRI to make it more widely accessible to providers. The hope is that, as with transcranial magnetic stimulation, patients might receive something like a 20-minute treatment two times a week over a span of a few weeks and taper as symptoms improve.
The Power in Ultrasound
While ultrasound is one of the oldest imaging modalities, technological advances have driven a rediscovery and reinvention of its capabilities.
“Ultrasound therapy is becoming an increasing important part of treatment plans for multiple neurological disorders,” Englot said. “This multidisciplinary project aims to develop novel uses of this technology that can improve neuromodulation treatment in many populations.”
Caskey adds that future applications may involve delivering acoustically active drugs or even genes to different parts of the brain, something they are funded to explore in preclinical models.
“I’m so encouraged by how powerful this tool can be, because we can try it on a nearly unlimited number of targets, exploring which one is works better, and all without harm,” Caskey said.