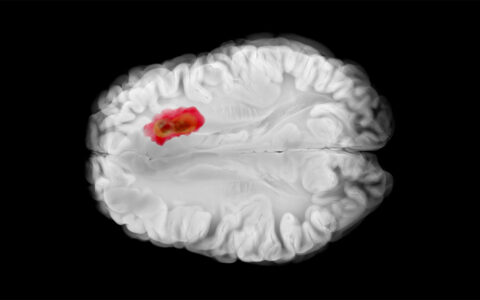
An acidic microenvironment creates the perfect home for malignant tumors. Extracellular calcification encourages tumor progression, as well as resistance to chemotherapy and immunotherapy.
Though alkaline therapy has long been imagined, it has not been realized due to the body’s demand for a pH range of between 7.35 to 7.45.
Now, in a remarkable development, a Vanderbilt research team led by Mohammed Noor Tantawy, Ph.D., Tantawy has created a novel drug nanoparticle – sulfonated polystyrene solution (NSPS) – that strategically attacks extracellular calcification.
“It’s been known for a long time that if we induce alkalosis in mammals, the tumors are going to die,” said Tantawy, a nuclear physicist turned radiologist at Vanderbilt University Medical Center. “But giving a patient something like bicarbonate causes alkalosis of the whole body, with side effects that can include kidney damage.”
Instead, NSPS breaks down hydroxyapatite (HAP), a key component of the calcification, raising the extracellular pH and dismantling this armor without affecting the body’s overall pH balance.
This breakthrough was reported in Cancer Medicine.
“Until now, nobody has devised any therapy that targets HAP, which is a key component of these calcified defenses and susceptible to destruction by our novel drug.”
“Until now, nobody has devised any therapy that targets HAP, which is a key component of these calcified defenses and susceptible to destruction by our novel drug,” said Tantawy, who was first author on the manuscript.
Preclinical research robustly supports his theory that the NSPS nanoparticle reverses acidosis in the tumor microenvironment, inhibiting tumor metabolism and growth, without adverse effects on healthy cells.
“NSPS appears to be purely the enemy of HAP produced by tumor cells,” he said. “Since the drug doesn’t target the tumor cells themselves, they don’t develop resistance to it.”
Defenses Foiled
HAP is a naturally occurring component of bone, but it is also manufactured in tumor cells and is associated with enhanced proliferation, progression and metastases, the researcher explained.
Once the tumor cell manufactures HAP, it migrates out into the external microenvironment around the tumor where it defends against T cells and threatening drugs like those used in chemotherapy and immunotherapy.
Tantawy postulates that NSPS binds to calcium on these tumor-associated HAP lattices, causing them to disintegrate into solution as calcium, phosphate and hydroxyl ions. Healthy tissue cells are unaffected.
“In essence, NSPS shocks the exposed tumor cells and sends them into a kind of metabolic hibernation,” Tantawy explained. “We observe them starting to die within 24 hours after NSPS injection. Importantly, without their shield, they become weak and potentially more vulnerable to established treatments.”
“We observe them starting to die within 24 hours after NSPS injection. Without their shield, they become weak and potentially more vulnerable to established treatments.”
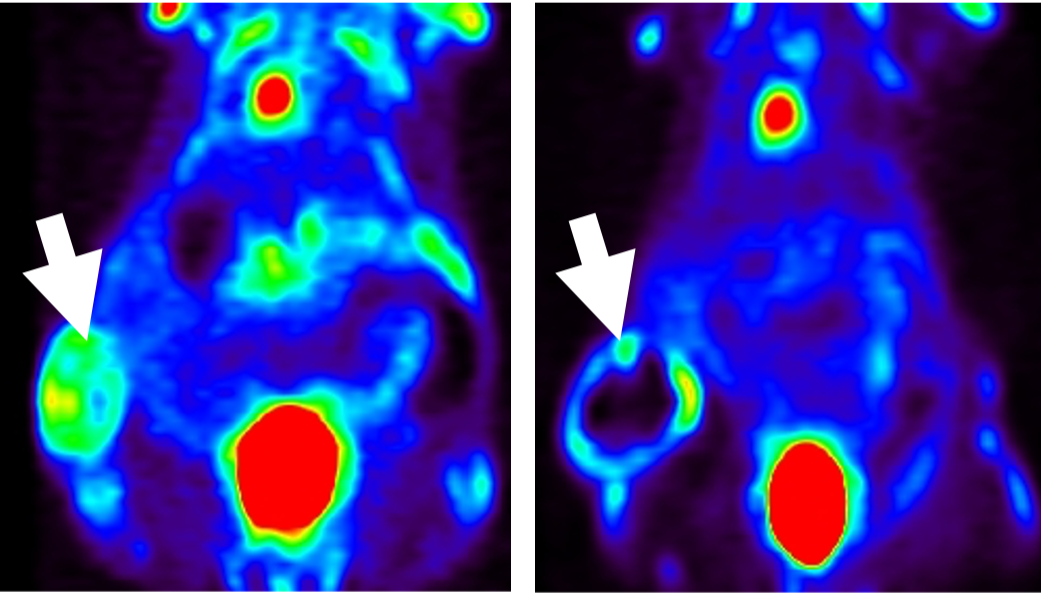
This is particularly promising for attacking cancers like triple-negative breast and ovarian cancer that tend to be highly drug-resistant. For example, in ovarian cancer, which is largely non-responsive to immunotherapy, the researchers say NSPS treatment triggers an unprecedented flow of T cells invading the tumors.
Tantawy says NSPS would work not only in these highly intransigent tumors, but in any tumor that has calcification in its extracellular matrix.
“We are now exploring other types of malignancies, including lung, prostate, pancreatic, and colon cancer,” he said. “So far, we have promising results. There are reports that infectious pathogens might also produce HAP, so we’re going to explore it as a potential therapy there as well.”
Compound Origins
Tantawy has spent the past decade testing different combinations of molecules at the Vanderbilt University Institute of Imaging Science (VUIIS) to find a compound that performs as NSPS does. He and his team use Raman spectroscopy to confirm the presence of hydroxyapatite, PET scans to validate the calcium presence and metabolic hibernation, and NMR to validate the changes in pH from acid to alkaline.
“The process has been what I would call an educated trial and error journey, trying different types of combinations until the day came when I could say, ‘Eureka! I think this is it!’” he said. “Along the way, I have worked with the most fantastic group of physicists, chemists and biologists at Vanderbilt, as well as Vanderbilt oncologists, who helped steer me to what was clinically important.”
Next steps for Tantawy are to optimize dosage and delivery methods and to develop practical aspects of producing NSPS. He is currently working with the Vanderbilt Chemistry Synthesis core to take the NSPS nanoparticle from a monomer in the lab to full-scale polymer of the optimal size, ready for clinical assessment.
“NSPS is large, about 55 kilodaltons, a size that is good in that it limits its interaction with bone, but is difficult to manufacture. We are working on optimizing NSPS as a smaller, but still potent and safe, compound to facilitate eventual translation to the clinic,” he said.