In deep brain stimulation (DBS), circuits are reset, modulated or redirected to drive symptom management in conditions including epilepsy, depression, obsessive-compulsive disorder, essential tremor, dystonia and Parkinson’s disease.
Across all of these, however, DBS has not yet qualified as a “disease-modifying treatment.”
For Parkinson’s, however, that paradigm might be changing, following a recent study conducted at Vanderbilt University Medical Center, in partnership with the Charité-Berlin University of Medicine in Berlin, that proposes a novel DBS treatment protocol for the treatment. This work provides beginning evidence that DBS applied in this fashion can be disease-modifying in Parkinson’s disease.
Vanderbilt Parkinson’s disease researcher Mallory Hacker, Ph.D., and Department of Neurology Vice Chair David Charles, M.D., coauthored a publication in the Annals of Neurology presenting findings from their randomized pilot study. In it, motor progression scores for five out of 14 study group participants with early Parkinson’s disease were the same or better two years after receiving DBS. The researchers used a software toolbox called Lead-DBS to analyze why these five participants may have benefitted so dramatically.
“It potentially becomes the first therapy ever discovered that would slow or stop the progression of motor symptoms.”
Hacker led this research, finding that confining stimulation to nerve fibers known to promote healthy motor pathways may be key to slowing or stopping progression. Now, a larger, multicenter study is in the planning stages to see if a greater swath of patients may benefit under this new, refined approach.
“If Dr. Hacker’s discovery is proven true in follow-up studies, it will transform the way that DBS is done for Parkinson’s disease,” Charles said. “Even more exciting is that it potentially becomes the first therapy ever discovered that would slow or stop the progression of motor symptoms.”
DBS Mechanism of Action
Parkinson’s disease is characterized by intracellular accumulation of misfolded forms of the protein α‐synuclein and progressive loss of dopaminergic neurons in the substantia nigra. While both the substantia nigra and subthalamic nucleus are key nuclei affected in Parkinson’s disease, the latter is a common target in DBS because of its role in movement control, the researchers explained.
Declining motor function is thought to be due to early death of dopaminergic neurons, yet there is little knowledge regarding how this contributes to biochemical or other changes, such as loss of brain-derived neurotropic factor (BDNF) and other neuroprotective agents.
With confirmation of these study outcomes, the question of whether and how DBS works to slow or prevent neuronal death may be front and center.
Studies of DBS-driven changes in BDNF and other neuroprotective agents have shown some selective regional impact but no overall change in BDNF levels. Though chronic DBS induces gradual reorganization of neuronal circuits through enhancing synaptic plasticity and neurogenesis, no one has yet connected the dots by demonstrating how DBS might slow motor progression in patients with Parkinson’s disease.
Minding the Colors
The team’s discovery was unexpected, since there had not been, to date, any clear path to prevention, remission, or cure.
“We weren’t expecting to see stopped and even reversed motor progression, and that raised the question of what was different in those five patients.”
“We weren’t expecting to see stopped and even reversed motor progression, and that raised the question of what was different in those five patients,” Hacker said. “Given what we already know about how DBS location affects patient outcomes, we wanted to explore relationships between where stimulation was applied, the brain connections that might be involved, and motor progression in the only cohort of patients to receive DBS in early-stage PD.”
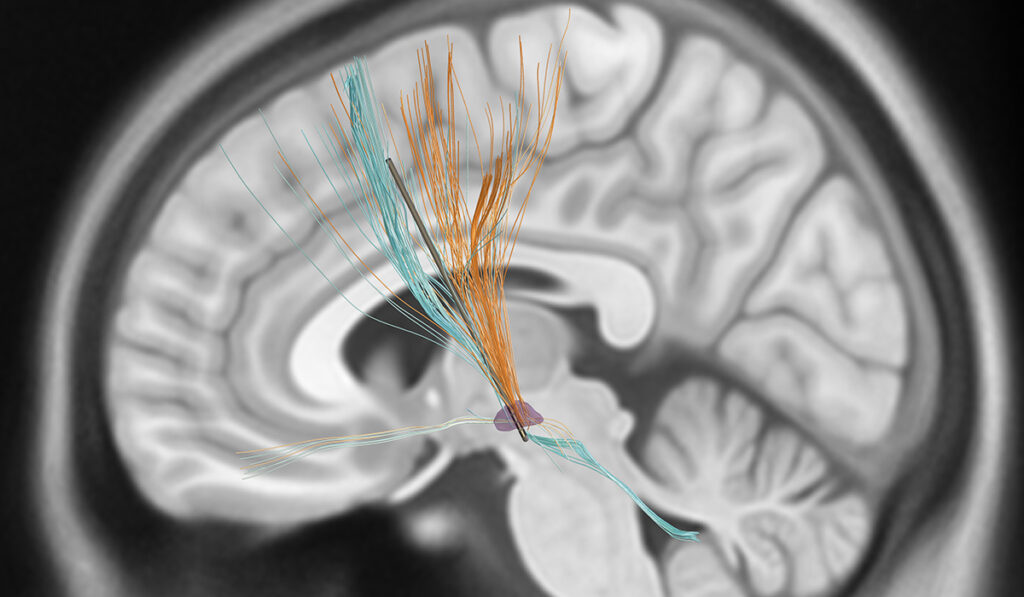
To do this, Hacker used brain scans collected as part of DBS surgery and a map of brain connectivity to delineate the fiber tracts, depicted as orange or blue post-imaging to denote, respectively, positive or negative associations with motor progression.
If confirmed in future studies, this approach could be used to build a template for future DBS implants. With such a map in hand, a neurologist, working with the neurosurgeon, could focus on implanting and programming the positive tracts to be stimulated, and avoid the negative tracts, Hacker said.
New electrode technology is another key to making this delineated stimulation possible by providing multiple levels of directional contacts on the leads. For the first time, current can be directed out from one of three zones on a 360-degree axis. Previously, it emanated from the contact spherically, with only the stimulation volume controllable.
Expanding the Frontier
The researchers have published long-term data from the trial showing fewer motor complications among those who received early DBS. Learning whether the team’s discovery, if replicated, extends to patients with more advanced Parkinson’s disease is the next frontier to explore.
“Currently for Parkinson’s, there is no medication, surgery, or biologic that slows down the relentless degeneration that is going on in the brain. Everything we have is helping the symptoms for a few hours at a time,” Charles said.
“What Dr. Hacker is onto is a way of programming the stimulation so that it actually could become disease-modifying. If we can understand the mechanism for this, maybe helping patients who are at other stages is possible.”