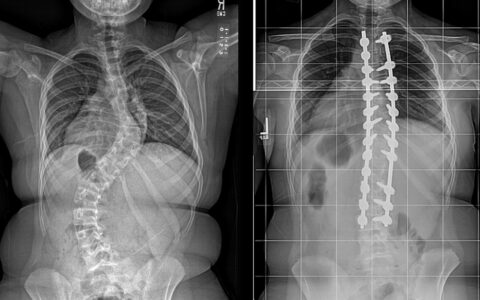
Jonathan Schoenecker, M.D., Ph.D., and his research team have completed a murine model study demonstrating how spinal fusion begins and progresses, and their findings constitute a sharp departure from the historical understanding of this process.
“We were asking the questions, “What is the biology of how the spine fuses, and what bone graft best promotes fusion?” said Schoenecker, a researcher and pediatric orthopaedist at Monroe Carell Jr. Children’s Hospital at Vanderbilt.
What they found exceeded the boundaries of these questions.
Using reporter mice, Schoenecker has identified the stem-cell-rich periosteum as the source of recruited cells – not bone marrow, as conventionally believed. These pluripotent cells become chondrocytes. Healing then proceeds through endochondral ossification, mediated by transdifferentiation of individual chondrocytes into osteoblasts.
POSNA Surprise
Schoenecker presented these findings at the 2023 Pediatric Orthopaedic Society of North America (POSNA) annual meeting. If their mouse model demonstration is confirmed in children, he says there are direct and dramatic implications for surgical approaches to the spine, with a particular impact on correction of severe spine deformities like scoliosis.
Monroe Carell pediatric orthopaedic surgeon Craig Louer, M.D., who was not involved in the research but attended the POSNA presentation and performs several complex scoliosis surgeries each year to straighten young spines, believes this better understanding of the biological mechanisms will open doors to new ways of thinking.
“While it is a bit too early to know, this research may not only change how we prepare a fusion bed but also improve on our graft materials,” Louer said.
Alignment with Prior Work
Schoenecker has spent his career establishing evidence about the nature of bone and cartilage. From his recent and ongoing work on how children heal from Perthes disease, which results from blood-deprivation to the femoral head, he produced evidence that that osteoblasts and chondrocytes appear capable of undergoing transdifferentiation, one to the other.
This recognition has informed treatment of Perthe’s disease by dictating that the moldable cartilaginous femoral head needs to be kept round through healing, so that it fits properly into the acetabulum, he explained.
Periosteum as Source
Turning to the spine, Schoenecker first set out to identify the progenitor cells used in the fusion process. To do this, he used a technology through which specific cells of the reporter mice are labelled with a fluorescent dye that lights up when they are on the move.
“Early on, we saw cells light up red on the posterior of the spine, not in the bone marrow, indicating that the periosteum is the source of these recruited cells.”
“Early on, we saw cells light up red on the posterior of the spine, not in the bone marrow, indicating that the periosteum is the source of these recruited cells,” he said. “So, basically, we were seeing this explosion of activated pericytes.”
This has tremendous implications for surgical techniques, he explained.
“This work indicates that we should be protecting the stem cell-rich periosteum.”
“This work indicates that we should be protecting the stem cell-rich periosteum,” Schoenecker said. “Sparing the removal of bone would also mean a less painful surgery with less blood loss.”
Louer noted that periosteal stripping followed by gouging or burring of the exposed bone to prepare a fusion bed has been a constant in spine fusion work since its inception.
“But when this work was presented, we spine surgeons all lined up at the microphones trying to find out more and whether this work should alter our surgical approach,” he said.
More Telltale Lights
The second thing Schoenecker explored was what happened to those cells labeled chondrocytes as bone healing progressed.
“Classically, it is described that healing occurs through intramembranous bone formation, where the stem cells differentiate into osteoblasts to directly make bone,” he said.
“Chondrocytes are sufficient by themselves to fuse a spine.”
Fourteen days post-fusion surgery, the researchers observed robust formation of chondrocytes in the fusion bed and went on to confirm their type through histological analysis.
“This supported our hypothesis that spine fusion occurs through an endochondral process instead of one where osteoblasts are formed directly from stem cells,” he said.
Following these same cells as fusion progressed, he found that at 42 days, when formation was complete, the majority of the osteoblasts in the new bone retained the chondrocyte marker, the telltale sign of their origin.
Schoenecker also wanted to determine if chondrocytes alone could fuse a spine. He placed chondrocytes at the site of the desired fusion and observed robust new bone formation in comparison with sham and iliac crest bone graft.
“These data together affirm our hypothesis that chondrocytes are sufficient by themselves to fuse a spine,” he said.
More Surgical Implications
“The potential clinical implications of this work are, first, to respect the periosteum, as it is the main source of stem cells. Second, the choice of graft should support endochondral ossification, making chondrocytes themselves good cellular bone-graft material,” Schoenecker said.
Both he and Louer foresee benefits for a broader population.
“Progression in this research could lead to applications for other spine and orthopaedic procedures where fusions are more difficult to obtain, such as the cervical spine or a lumbar spondylolisthesis,” Louer said.