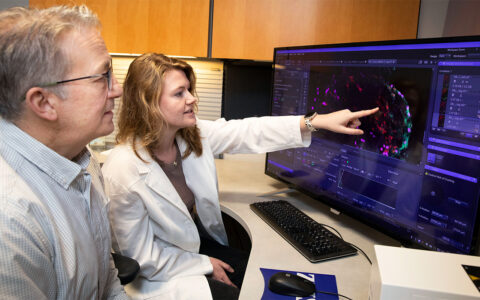
Featured in the Vanderbilt Eye Institute Impact Report 2024. Peruse the rest of the Impact Report here.
A cohort of beagles with inherited glaucoma is providing clues about neurodegeneration in retinal ganglion cells that contribute to glaucoma in humans.
In 2012, researchers at Vanderbilt Eye Institute and the University of Florida, Gainesville, identified a variant of the gene ADAMTS10 as the mutation that causes glaucoma in the beagle cohort. Those findings were later independently verified in other canine models, and ADAMTS genes have been associated with human glaucoma.
Recently, a Vanderbilt team led by John Kuchtey, Ph.D., and Rachel Kuchtey, M.D., Ph.D., found ADAMTS10 to be a glaucoma-causative factor in both mouse and zebrafish models. The Kuchteys led the original ADAMTS10 discovery team, which got its start in the late 1970s.
“We’re still following through with our original work with the beagles,” said John Kuchtey, a research associate professor at Vanderbilt. “Finding a disease gene is just scratching the surface.”
“We’re still following through with our original work with the beagles. Finding a disease gene is just scratching the surface.”
Rachel Kuchtey, a clinician-scientist and glaucoma specialist at Vanderbilt Eye Institute, said the findings have relevance specific to human glaucoma.
“It’s really the pathways that these molecules take that may be more relevant than one single player,” she added. “Understanding how they contribute to disease pathogenesis will enable us to identify novel treatment strategies.”
Critical Role for ADAMTS10
The core of elastic fibers found in the optic nerve is surrounded by a sheath of fibrillin microfibrils. The ADAMTS10 protein is involved in normal formation of the microfibrils, which store latent transforming growth factor beta (TGF-β), a family of signaling proteins.
TGF-β promotes the development of retinal ganglion cells and is also known to play other roles, John Kuchtey said. In glaucoma, abnormal microfibrils can lead to dysregulation of TGF-β signaling, a potential genesis of the disease.
Mutations in ADAMTS10 have been shown to cause the autosomal recessive form of Weill-Marchesani syndrome in humans, with lens thickening and angle closure glaucoma as common complications. The dominant form of Weill-Marchesani syndrome is caused by mutations in fibrillin-1. Marfan syndrome is also caused by fibrillin-1 disruptions, Kuchtey noted.
“The question we’re asking is: What is the effect of ADAMTS10 on these microfibrils and how does it impact TGF-β signaling?”
Preserving Ganglion Cells
Microfibrils contribute to the mechanical function of the lamina cribrosa, where the retinal ganglion cells exit the back of the eye. Researchers believe that biochemical changes in the lamina cribrosa and surrounding tissue cause impingement of the retinal ganglion cell axons. This leads, in turn, to reduced delivery of growth factors, causing degradation and cell death.
In their latest study, the Kuchtey team introduced into mice the ADAMTS10 mutation found in beagles. They found abundant ADAMTS10 in the retinal ganglion cell bodies and in optic nerve axons. However, in a surprise result, it was not associated with microfibrils.
“This was an unexpected finding, since previous studies of ADAMTS10 focused on interactions with microfibrils,” John Kuchtey said. “Our results showed that in these tissues that essentially define glaucoma, ADAMTS10 has a previously unknown function, independent of microfibrils.”
“Our results showed in these tissues that essentially define glaucoma, ADAMTS10 has a previously unknown function independent of microfibrils.”
The researchers found that mice with the glaucoma-causing mutation had a marked reduction of TGF-β activity as they developed, suggesting a novel role for ADAMTS10 in managing cytokine, known to be an important regulator of extracellular matrix structure.
“There are other ways to regulate TGF-β; we’re looking at other proteins,” Kuchtey said. “One of these is thrombspondin-1 (TSP1), which displaces fibrillin and allows active TGF-β to bind to its receptor and activate signaling.
“Our hypothesis is that ADAMTS10 does a similar thing.”
Future Implications
Not all patients with elevated intraocular pressure (IOP) develop glaucoma, and some patients with apparently normal IOP continue to progress. John Kuchtey said a major unmet need is to identify other targets for glaucoma treatment.
“Modulation of TGF-β signaling could offer a new approach to glaucoma pathogenesis that directly addresses the changes in the properties of the extracellular matrix in the optic nerve tissue,” he said.
Another translational focus in the Kuchtey lab is investigation of the role of sartan drugs in preventing or slowing the progression of glaucoma. Sartans, such as losartan, are angiotensin II type I receptor antagonists commonly used to treat systemic hypertension. Recently, sartans have been shown to be an effective treatment for Marfan syndrome.
For these studies, the team carried out retrospective cohort studies of glaucoma patients to determine if sartans protect against initial development of glaucoma or slow progression for those previously diagnosed.
“If we can use existing therapeutics to slow retinal ganglion cell degeneration in glaucoma, we will be that much farther along in our work to treat this blinding eye disease.”