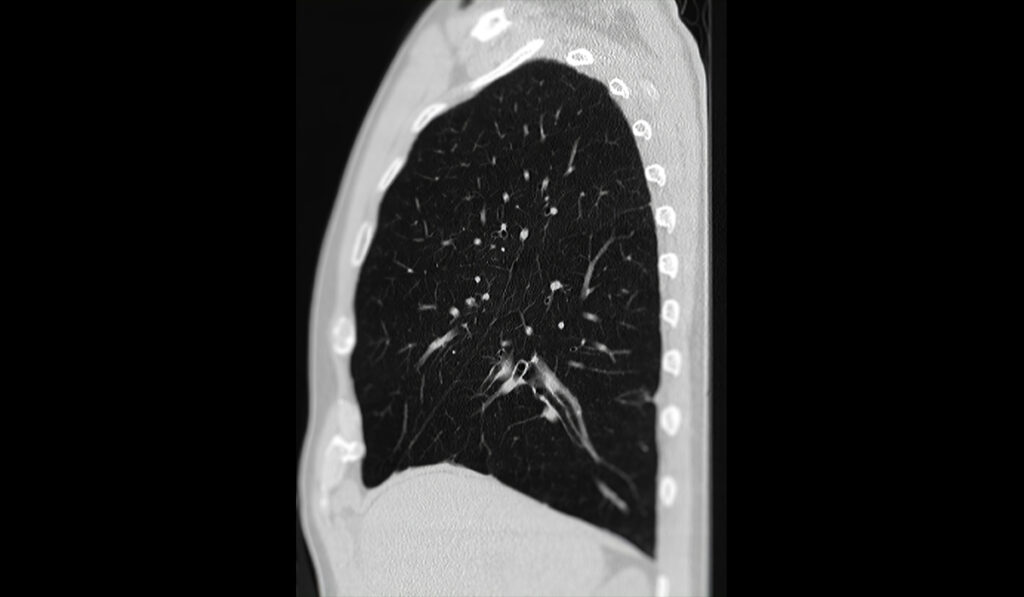
With low-dose CT lung screenings now in reach for more patients, rates of lung cancer-specific mortality have dropped significantly – by 20 percent in some high-risk groups.
Radiologists, however, are now dealing with an unwanted side effect: the added expense and workload that occurs when lifesaving low-dose CT scans also identify nodules of indeterminate cause that require further investigation.
Although 96 percent of these incidental findings are eventually determined to be benign, when flagged, an expensive PET scan or an invasive biopsy may be warranted to rule out cancer, said Fabien Maldonado, M.D., professor of medicine and thoracic surgery at Vanderbilt University Medical Center.
Millions of lung nodules, incidentally or directly through screening, are detected every year in the United States. Each scan is evaluated by a radiologist, so more scans overall mean radiologists are working at a faster pace and looking for ways to build efficiency into the process.
Agreed on by much of the biomedical community is the need to come up with a non-invasive means of categorizing the growing flood of indeterminate nodules that appear on CT scans.
The subject is a natural focus for researchers involved with VUMC’s comprehensive lung cancer program. At the leading edge of this effort is a proposal to mold artificial intelligence (AI) capabilities into reliable and pragmatic tools for medical use.
Maldonado, a national leader in AI solutions for lung nodule discrimination, is investigating a deep-learning system that could leverage data from CT scans and electronic health records to flag suspicious nodules among those potentially misidentified as indolent or benign. He and his colleagues Tom Lasko, M.D., Ph.D., an associate professor of biomedical informatics, and Bennett Landman, Ph.D., chair of the Department of Electrical and Computer Engineering, are handling portions of the project’s development.
Untenable Scan Volume
The large volume of scans needing attention is compounded by findings of cancer of minimal clinical significance, Maldonado said. Over-diagnosis and over-treatment of adenocarcinoma, the most common form of lung cancer, is especially challenging.
Patients are more likely to die with malignant but indolent nodules than from them, Maldonado said.
“The diagnostic difficulty cuts both ways, though, with many aggressive cancers initially thought to be insignificant,” he said. “It can take a very experienced lung-nodule expert to identify those that need follow-up, biopsies and surgical resection.”
Clinical Signatures
Probabilistic deep learning is one avenue for improving predictions of malignancy in indeterminate nodules.
In a novel way, Lasko mined EHR data for all factors that could pertain to risk, organizing the variables into learned clinical signature patterns, which are more powerful and interpretable than the raw variables, either on their own or when combined with images.
“Thousands of relevant clinical signatures are found distributed among lab tests, billing codes, medications, and demographics,” Lasko said. “We’ve found patterns that look like undiagnosed lung cancer or undiagnosed bladder cancer that increase the probability of malignancy – or patterns of infection that decrease it.”
The model is queried after it is fed data from everyone with lung disease, not just cancer. This broader query leverages the statistical strength of the data set of 270,000 records, Lasko said. When compared with actual patient diagnoses, the data yielded a high area under the curve (AUC) of 0.79.
Power in Data
Landman’s role has been to apply new algorithms to interpret data from hundreds of thousands of CT images to further enrich the pool of predictive information.
“Instead of looking at them as images, we look at them as data,” Landman said. “And then we do a whole host of advanced modeling to determine whether these nodules are malignant or not.”
“We now have internally validated radiomics models, as well as a combination model that is approaching the accuracy of expert-based or other AI model predictions of malignancy,” Landman said.
The model using both images and clinical signatures produced an even higher AUC of 0.82, approaching the performance of lung nodule .
Deeper Dive
If deep learning is better at predicting a tumor’s trajectory than unassisted readings, its use will save time for radiologists and nurse navigators.
“The model is acting like a radiologist who doesn’t just look at the smudge on the film but wants to know what’s going on with the patient, who ordered the film, and why they ordered it,” Maldonado said. “The unsupervised learning takes all of that into account.”
“The model is acting like a radiologist who doesn’t just look at the smudge on the film but wants to know what’s going on with the patient, who ordered the film, and why they ordered it.”
At the same time, he cautions, that there is no model of this concept ready to launch. Many questions remain.
“Are there signs that wouldn’t have been concerning except in the context of this finding… How do we integrate the model’s predictive power into a patient-centric learning approach? This is all still evolving,” Maldonado said.
A randomized controlled trial is being planned to compare their combination model with standard diagnostic approaches and evaluate risk-stratification success.
“The holy grail would be to run these on the EHRs for every patient in the hospital who has had a chest CT for any reason,” Maldonado said. “This approach could improve predictive stratification for almost any thoracic condition.”