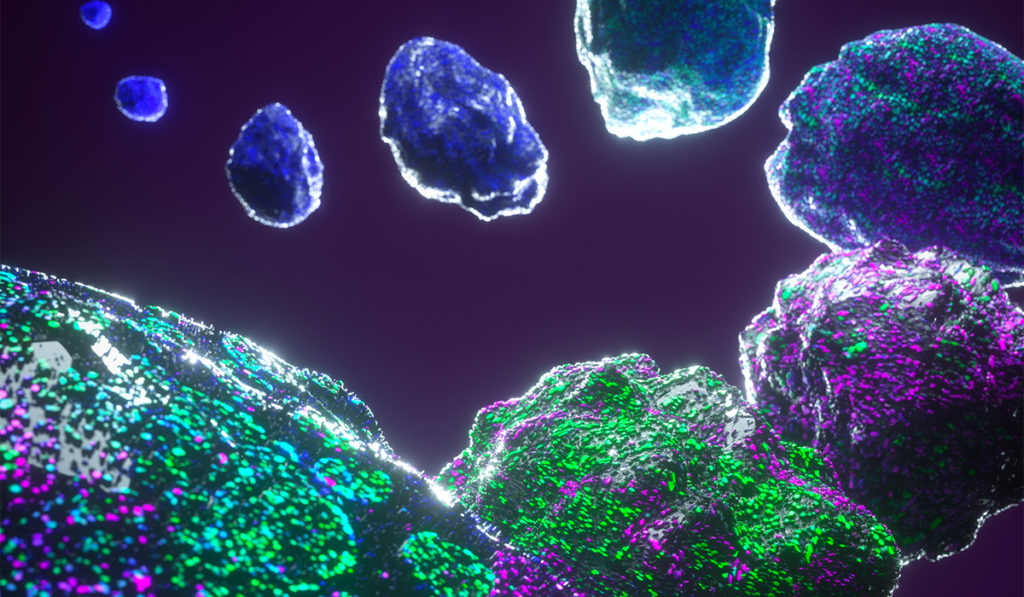
Triple-negative breast cancer (TNBC) tumors that express high levels of the oncogene MYCN are sensitive to combined BET (bromodomain and extraterminal motif) and MEK (mitogen-activated protein kinase) inhibition, according to new research published in Science Translational Medicine.
“New therapies for triple-negative breast cancer will address unmet medical needs for women and men with this type of breast cancer. Our discoveries provide pre-clinical data for clinical trial investigation of the potential utility of MEK and BET inhibitors in advanced triple-negative breast cancer,” said Jennifer Pietenpol, Ph.D., director of the Vanderbilt-Ingram Cancer Center and lead author on the study.
“Our discoveries provide pre-clinical data for clinical trial investigation of the potential utility of MEK and BET inhibitors in advanced triple-negative breast cancer.”
A Role for MYCN in TNBC
TNBC tumors lack high-frequency driver alterations that are therapeutically targetable and have highly heterogeneous intratumoral cell populations, making the development of treatment strategies a challenge.
To identify a potential therapeutic target, Pietenpol and colleagues focused on the oncogene MYCN, which increasing evidence suggests plays a role in breast cancer recurrence and metastatic spread. For other tumors, like neuronal and neuroendocrine tumors, aberrant MYCN expression has been reported to be a driver of tumor growth.
Utilizing data from The Cancer Genome Atlas (TCGA), the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC), the TNBC587 dataset, as well as 344 TNBC tumor samples from Vanderbilt University Medical Center and US Biomax, the authors report heterogeneous expression of MYCN transcripts and MYCN protein in a substantial number of TNBC tumors.
“MYCN expression in TNBC was found to be comparable to MYCN expression in neuroendocrine-castration resistant prostate cancer, and between 45 and 64 percent of the TNBC tumor samples assessed had heterogeneous MYCN protein expression,” said Johanna Schafer, Ph.D., first author on the study and a research staff scientist at Vanderbilt University Medical Center.
Consistent with the heterogeneous nature of TNBC tumors, the authors also report heterogeneous expression of MYC, with most of the tumor cell nuclei robustly expressing either MYCN or MYC, but not both.
Inhibiting MYCN
A high-throughput screen of 158 drug compounds from the National Cancer Institute U.S. Food and Drug Administration (FDA)-approved oncology drug library identified BET inhibitors (BETis) that were effective at inhibiting cell growth in MYCN-expressing tumors.
BETis are under early-stage clinical development and target the bromodomain (BRD) family of transcriptional regulators. One member of the family, BRD4, regulates the transcription of MYCN and occupies genes and enhancers targeted by MYCN.
“Whereas previous studies have focused on BRD-mediated targeting of MYC, we show that TNBC tumors are heterogeneously composed of MYC- and MYCN-expressing cells and MYCN-expressing cells have differential sensitivity to BETis in select tumor cells and model systems,” the authors wrote.
While BETis caused levels of MYCN to decrease, they also caused MYC levels to increase. To target both MYC isoforms and overcome potential mechanisms of resistance, combination drug treatments were explored. Combining BETi with the MEK inhibitor (MEKi), trametinib, was shown to cause a synergistic inhibition of tumor cell viability in both cell culture models and TNBC patient-derived xenograft animal models.
Moving into Clinical Development
TNBC represents about 15 percent of breast cancer cases. Treatment for many patients is limited to chemotherapy, and the five-year survival rate for metastatic TNBC is 11 percent.
Based on these promising preclinical data, Pietenpol and colleagues propose the clinical development of combination BETi and MEKi for patients with advanced TNBC.
“As a next step, our research team is proposing the further development and clinical trials of this combination therapy,” Pietenpol said.
While BETis are currently under clinical development, a number of MEKis have already been approved by the FDA.