Providers caring for patients with sickle cell disease (SCD) walk a tightrope to determine how aggressively to treat their condition. Available treatments are conservative medical therapy for those at lowest risk for stroke, or more aggressive blood transfusion or stem cell transplantation for patients at higher risk. While screening procedures exist to triage children for different therapies, no established screening procedures exist for adults. This is a fundamental limitation, as patients are living longer into adulthood.
Biophysicist Manus Donahue, Ph.D., and pediatric neurologist Lori Jordan, M.D., both of Vanderbilt University Medical Center, are developing new imaging tools to identify how the brain utilizes oxygen in patients with SCD.
“The goals of this work, which are made possible by collaborations from specialists in neurology, hematology, and radiology, are to develop fast and non-invasive tools to quantify individualized stroke risk, to use this information to triage patients for personalized therapies, and to confirm that the therapies are successful by measuring their impact on tissue health,” Donahue said.
“The goals of this work are to develop fast and non-invasive tools to quantify individualized stroke risk, to use this information to triage patients for personalized therapies, and to confirm that the therapies are successful by measuring their impact on tissue health.”
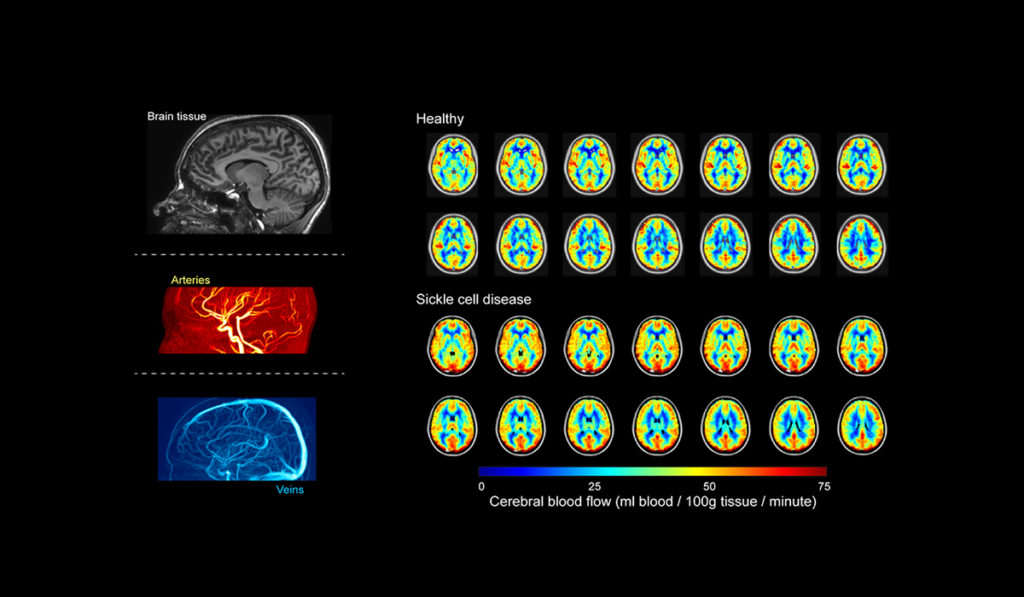
Oxygen Extraction Fraction
SCD is an inherited blood disorder that affects hemoglobin’s oxygen-carrying capacity and places patients at risk for stroke and other organ failures. One promising area of the team’s research uses a novel measure of brain oxygen extraction fraction (OEF) to capture how well the brain is compensating for the disease.
“The OEF describes the total amount of oxygen consumed by the brain relative to the amount delivered, and we can quantify this quickly and without injections using MRI. We do this by tracking the amount of oxygen and blood entering the brain through the arteries and the amount of oxygen leaving the brain through the veins,” Donahue said.
When the balance of oxygen consumed and delivered is not maintained, it signifies that oxygen delivery is not meeting the demands of the brain tissue and a stroke, or in fact, may be possible, Donahue explained.
Added Jordan, “We have shown that higher OEF is associated with higher rates of prior stroke in adults and children, and also that OEF can normalize to healthy levels after stem cell transplant, which is an emerging therapy for individuals with SCD that has curative potential.”
“We have shown that higher OEF is associated with higher rates of prior stroke in adults and children, and also that OEF can normalize to healthy levels after stem cell transplant.”
Researching OEF Accuracy
The ongoing goal of Donahue and Jordan’s research, which now includes MRIs on more than 250 participants, is to confirm the use of OEF as a predictor of new strokes by following patients over time. With Vanderbilt hematologists Michael DeBaun, M.D., and Adetola Kassim, M.D., the team is also demonstrating how OEF normalizes after emerging stem cell therapies. This could provide an improved screening tool for SCD patients and motivate its use as an endpoint in clinical trials that evaluate the efficacy of emerging therapeutics.
One of the most impactful outcomes of this work is that several of the technologies developed have been incorporated into the standard imaging protocols at Vanderbilt for SCD patients.
“Sickle cell disease is an especially exciting area lately, as promising curative therapies are being developed which stand to permanently improve the lives of patients. Confirming how these therapies affect brain health, and also which patients require these therapies, should be possible as a result of this work,” Donahue said.