On May 24, following the promising results of the REACH1 trial of ruxolitinib (brand name Jakafi®) for steroid-refractory acute graft-versus-host disease (aGVHD) and priority review by the FDA, ruxolitinib became the first drug approved for treatment of these patients.
Madan Jagasia, M.D., chief medical officer at Vanderbilt-Ingram Cancer Center and lead investigator of the REACH1 trial, calls the FDA approval “a major step forward for the scientific field of acute GVHD, which will open up a new era of clinical trials for this steroid-sparing approach.”
Jakafi® is marketed by Incyte in the U.S. and Novartis outside the U.S. It was previously approved for certain patients with polycythemia vera and myelofibrosis. Incyte worked with researchers to attain Breakthrough Therapy Designation and Orphan Drug Designation to expand its use to this small but medically vulnerable population.
REACH Studies
The REACH1 trials investigated if Jakafi® could favorably impact the outcome of patients with steroid-refractory aGVHD. The REACH1 phase 2 study was a single-arm, multicenter trial that tested Jakafi® in combination with corticosteroids. In the 71 patients refractory to corticosteroids, the overall response rate at 28 days was 55 percent.
“The side-effects of Jakafi® are consistent with what we expect to see in this population of immunosuppressed patients and most commonly include low blood cell counts and infection risk.”
“The side-effects of Jakafi® are consistent with what we expect to see in this population of immunosuppressed patients,” Jagasia says, “and most commonly include low blood cell counts and infection risk.”
A Monumental Step in Treatment
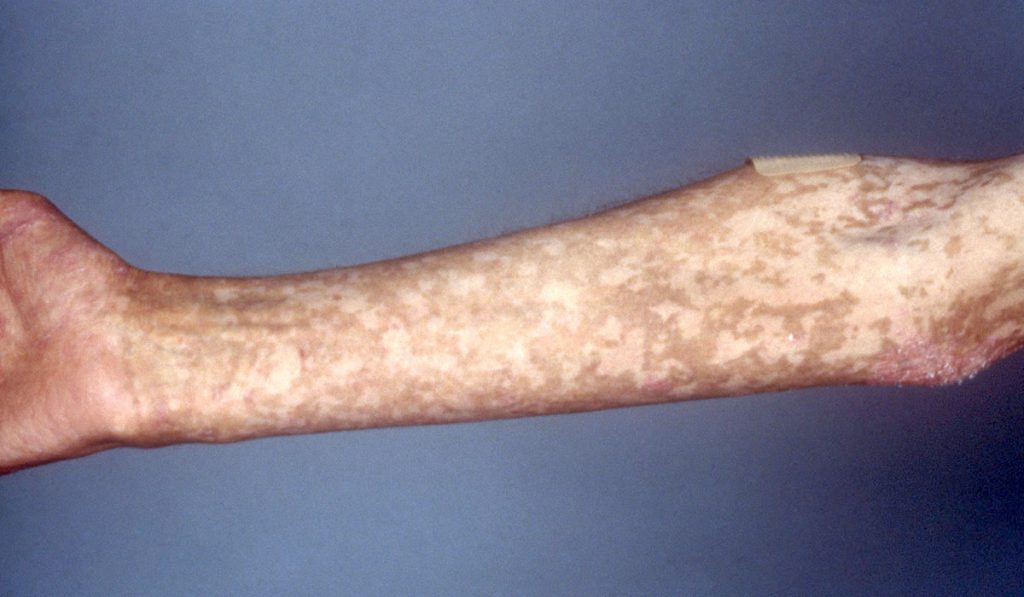
Seventy percent of patients who have undergone a donor stem cell transplant, a highly curative procedure, develop aGVHD. Only about 50 percent of these patients respond to corticosteroids. Prior to the approval of Jakafi®, these patients had very limited options and experienced poor outcomes. They are prone to consequences of immune dysregulation and suffer from significant infections. In many cases, especially of lower gastrointestinal aGVHD, nutrition is compromised and patients suffer from significant abdominal pain.
For these individuals, who face high mortality risk, Jakafi® approval is monumental, says Jagasia.