Beta cell dysfunction is central to type 1 and 2 diabetes, yet these insulin-producing cells are notoriously difficult to isolate and study. Many isolation techniques require in vitro labeling of fixed cells. This makes it currently impossible to measure a patient’s live beta cell function, or overall quantity, to inform treatment regimens.
In a recent article published in Cell Metabolism, researchers at Vanderbilt University Medical Center describe a new way to measure insulin-producing beta cells in vivo. After validating NTPDase3 (ectonucleoside triphosphate diphosphodydrolase-3) as a cell surface biomarker for beta cells, the group leveraged the biomarker in several experimental applications including quantifying beta cells in mouse models of diabetes.
If the results translate clinically, NTPDase3 could help researchers detect live beta cells in humans. “If someone could measure beta cell mass non-invasively in humans, that would change the way we understand, diagnose and treat diabetes,” said senior author, Alvin C. Powers, M.D., the Joe C. Davis Professor of Biomedical Science in the Vanderbilt University School of Medicine and director of the Vanderbilt Eskind Diabetes Clinic.
A New Biomarker for Beta Cells
NTPDase3 is a membrane-bound enzyme found in the endocrine islet of the human pancreas. It modulates extracellular nucleotide levels and therefore intracellular nucleotide signaling.
There is very limited information about NTPDase3 protein distribution in humans. The group decided to study the enzyme given “NTPDase3 is detectable by immunohistochemistry in pancreatic islets of both humans and rodents,” they wrote.
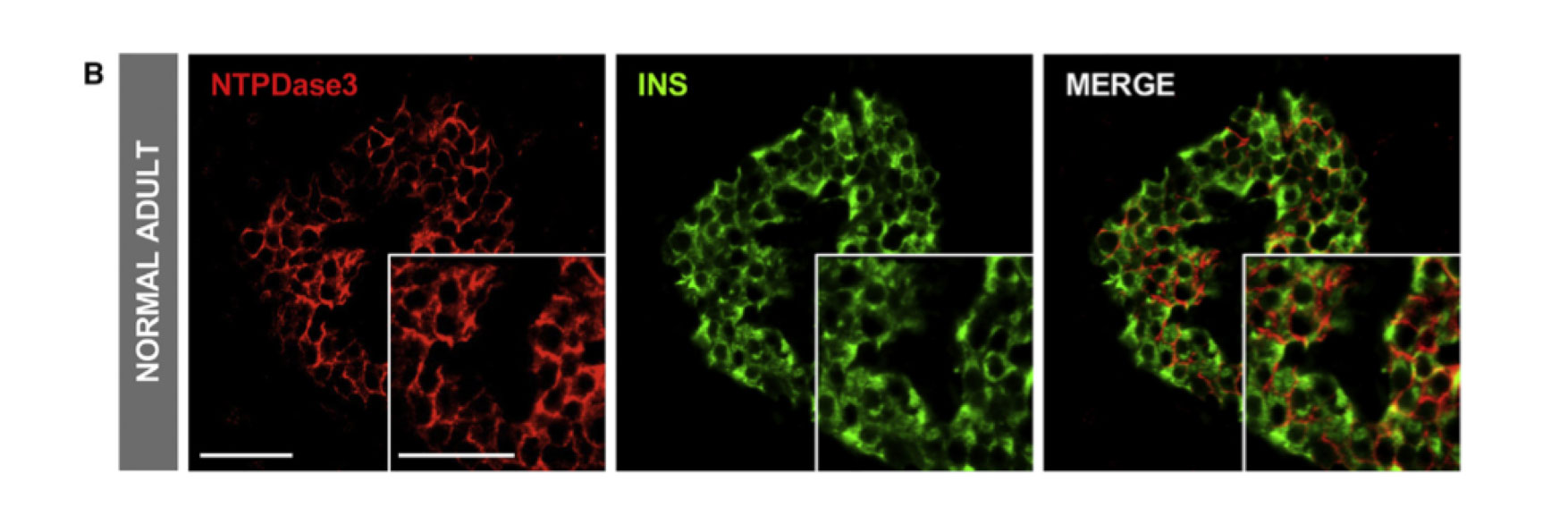
In pancreatic tissue sections from human donors, the researchers found NTPDase3 only on beta — but not alpha — cells. It was stably expressed only on cells isolated from adults. NTPDase3 did not colocalize with other known surface proteins, making it an ideal candidate to specifically flag beta cells. RNA expression analyses confirmed NTPDase3 is specific to mature beta cells.
Isolating Beta Cells from Islets
To detect NTPDase3, Powers collaborated with researchers at Laval University in Québec, Canada, who developed an antibody to study NTPDase3 expression in the brain. First author Diane Saunders, Ph.D., postdoctoral fellow in Powers’ laboratory, and the team showed that the fluorescently labeled antibody could also be used to find beta cells in the pancreas.
The antibody also allowed the researchers to effectively and efficiently isolate beta cells from a heterogeneous population of live, dispersed human islet cells.
The antibody also allowed the researchers to effectively and efficiently isolate beta cells from a heterogeneous population of live, dispersed human islet cells. The antibody was successful in flow cytometry experiments using cells from type 1 and 2 diabetics, and healthy donors.
Moving Experiments In Vivo
“Since the NTPDase3 antibody allowed the sorting of live human beta cells, we then tested whether it could detect human beta cells in vivo,” wrote the authors. They transplanted human islets beneath the eye or kidney capsule of immunodeficient mice, waited four weeks, then went to work with the antibody to look for beta cells.
The researchers injected the mice with the (fluorescently-conjugated) antibody, then removed the islet transplants. Immunohistochemistry confirmed the antibody migrated to the transplants and specifically bound human beta cells. “Such results suggest that the epitope would be accessible in the native pancreas, which is important for imaging applications,” they wrote.
Potential for Translational Research
The study only looked at experimental applications, but the results suggest strong potential for translation to the clinic. Similar techniques could be used to measure beta cells in humans.
“The antibody directed to this protein should be a useful new reagent for beta cell sorting, in vivo imaging, and targeting,” wrote the authors. Added Powers, “Our results lay the foundation for quantifying beta cells in humans and understanding beta cell mass dynamics. Until now, this has not been possible.”