Among the many cell types contributing to progression of renal fibrosis in chronic kidney disease (CKD), tubular epithelial cells have received less attention compared to others such as myofibroblasts central to extracellular matrix production. Yet there may be key insights in better understanding the adaptive and maladaptive responses of epithelial cells such as the proximal tubule.
“The tubular epithelium is so central to injury. Its response can lead to changes in many other cell types including the myofibroblasts and inflammatory cells. It’s an important orchestrator of the fibrotic response in CKD,” says Leslie Gewin, M.D., assistant professor in the Division of Nephrology at Vanderbilt University Medical Center. One focus of Gewin’s current research is identifying the best possible responses to chronic injury within the tubular epithelia.
“Our hope is that by better defining adaptive versus maladaptive responses of the tubular epithelia to injury, we can better understand those processes and signaling molecules involved in fibrosis progression,” said Gewin.
Flow Cytometry to Characterize Epithelial Response
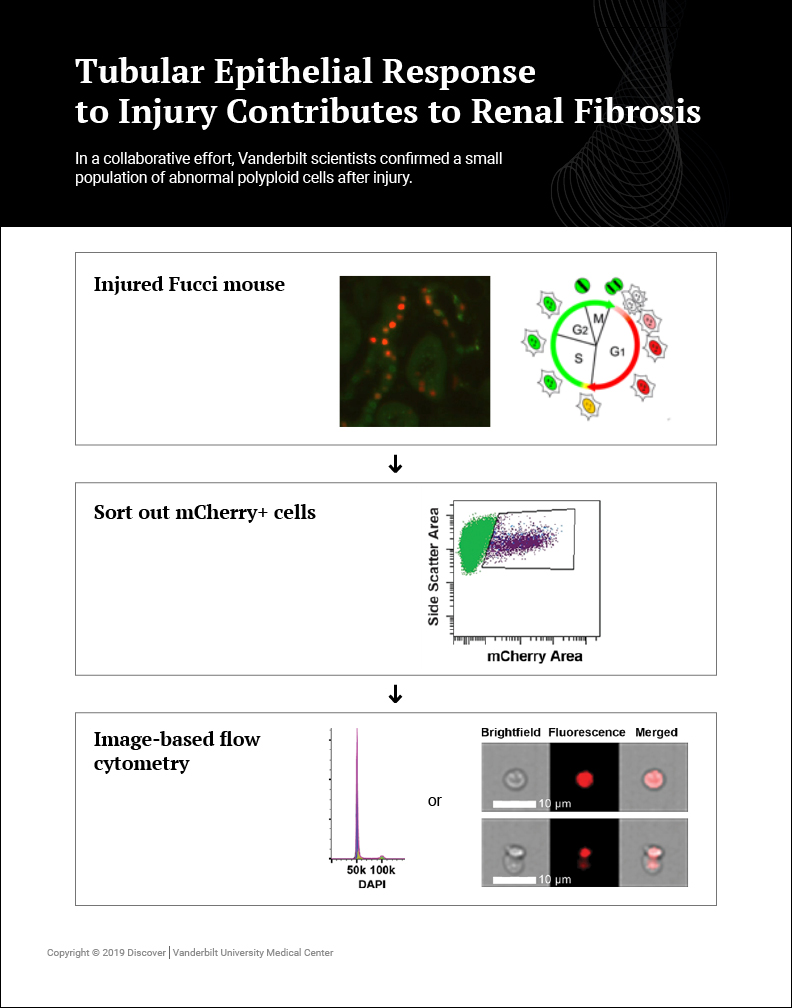
Gewin’s research team – part of the multi-laboratory Vanderbilt Center for Kidney Disease – is using flow cytometry to better characterize the cell cycle profile of injured cells in the epithelium such as the proximal tubule. Fluorescently tagging cells with a protein such as GFP provides a more accurate view of the cell-specific stages of the cell cycle. Gewin’s team uses image-based tools available within Vanderbilt’s Flow Cytometry Shared Resource in conjunction with a cell cycle reporter mouse to define cell cycle changes in models of CKD.
Collaborating with Brittany Matlock in the Flow Cytometry Shared Resource, Gewin’s team confirmed a small population of abnormal polyploid cells after injury. Polyploid cells replicate their DNA but do not divide, and further studies will determine whether they persist after injury and their functional significance. These studies were detailed in a recent publication in American Journal of Physiology Renal Physiology.
The publication includes a new dissociation protocol to prepare kidney tissue for analysis. Other researchers may benefit from the protocol that improves cellular yield and can be used to study any discrete cell population.
“This work would not have been possible without Vanderbilt’s Flow Cytometry Shared Resource. Brittany provided valuable guidance with the image-based FlowSight to visualize the cells with both brightfield and fluorescence, while simultaneously modeling the cell cycle,” said Gewin.
Interpreting Results
Gewin’s research shows changes in epithelial cell cycle can have important implications for the rest of the injured kidney. “Proliferating cells may be more susceptible to apoptosis in the hostile and hypoxic environment of CKD. Also, cells can become arrested at a later stage in cell cycle progression, and this growth-arrested cell can also have adverse effects in terms of promoting fibrosis,” Gewin said. “The injured proximal tubule cell can produce a large amount of profibrotic and proinflammatory cytokines that signal on surrounding inflammatory cells, the injured vasculature and fibroblasts to further promote kidney fibrosis and loss of function.”
A better understanding of beneficial epithelial responses to injury may lead to more targeted drug therapies promoting adaptive, rather than maladaptive, responses. “It is generally harder to target proteins that are beneficial, but oftentimes you can inhibit their inhibitors as a way of augmenting function,” Gewin said.
Next Steps for Research
Gewin is now studying exactly how the epithelial cell cycle changes under injury, and how new laboratory strategies are used to measure the process. Gewin also looks forward to expanding investigation into the role of growth factors in managing epithelial response. “In the past, we’ve looked at TGF-β and how it alters epithelial cells’ response to injury. I’m look forward to working with the Wnt/β-catenin pathway and understanding how it modulates the epithelial response to chronic kidney injury,” Gewin said.
“The ability to look at the transcriptome of each individual cell has the potential to really change our understanding.”
Emerging sequencing technologies, in particular single cell RNA-seq, may accelerate this research. “The ability to look at the transcriptome of each individual cell has the potential to really change our understanding of the differential responses of the many cell types within the kidney,” Gewin said.