Key Takeaways
- Two of six ovine subjects were stable and ambulatory through the seven-day trial period.
- Prior studies suggest that physical activity boosts ECMO patients’ transplant odds.
- Beyond chronic disease applications, the team is working to improve support for wounded soldiers with acute lung injuries.
The portable left ventricular assist device has transformed the lives of thousands of patients with left-sided heart failure, allowing them to return home while awaiting a heart transplant.
Unfortunately, there have been challenges to developing a similar device for patients with right-sided heart or chronic obstructive pulmonary disease-induced hypercapnic failure, hindrances to a respiratory equivalent of the LVAD.
Clotting and hemolysis are among obstacles to the respiratory support initiative. It has also confronted other hurdles, such as maintaining efficient gas exchange across a large surface area and managing the larger size and the footprint required for operation.
Working through these obstacles, a team led by Matthew Bacchetta, MD, MBA, surgical director of the Vanderbilt Lung Institute, is developing a lightweight, wearable pulmonary-assist system designed to keep patients alive and ambulatory. Advanced Respiratory Technologies Inc., an industrial partner of Bacchetta’s team, are designing and manufacturing the gas exchangers.
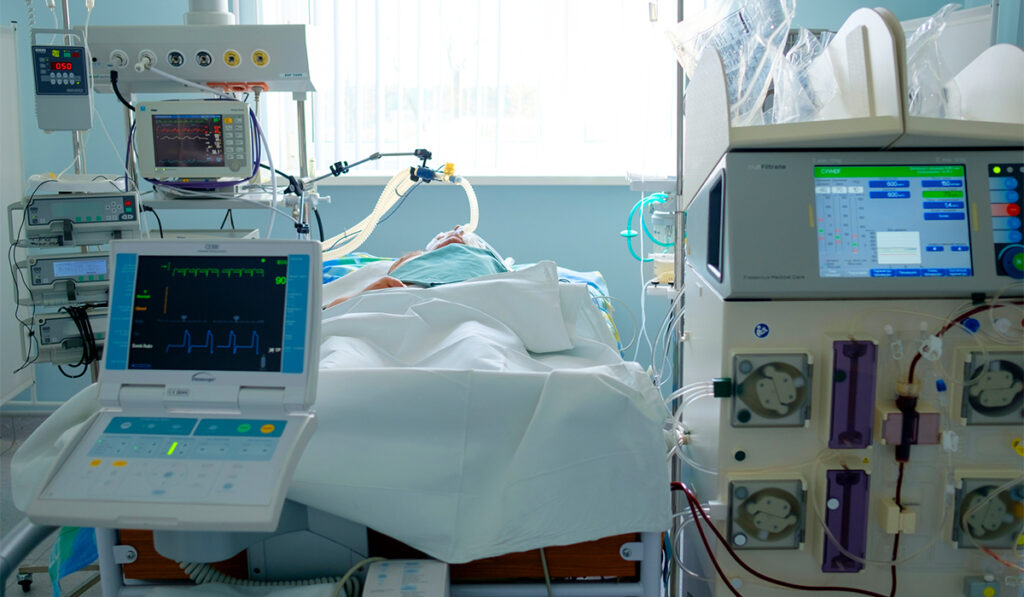
Bacchetta’s lab, co-directed by at Vanderbilt University Medical Center biomedical engineer Rei Ukita, PhD, recently tested the novel device in its latest series of ovine trials. They demonstrated that sheep with right-sided heart failure equipped with the device can remain stable and even ambulatory through a seven-day study period. The finding is promising for a large group of patients.
“There are so few donors for a pool of millions with end-stage lung disease,” Bacchetta said.
“Whether it’s due to COPD or interstitial lung disease or pulmonary hypertension, the overwhelming majority will never have an opportunity to receive a lung transplant. We need a better method to bridge patients to transplant – or to a destination therapy for those who are ineligible.”
ECMO Liberation
Prior studies suggest that physical activity boosts ECMO patients’ transplant odds, according to Ukita.
“We’ve used some creative means of getting ICU patients moving, but it takes heavy staff support,” Ukita said.
Added Bacchetta: “What we’re really trying to do is offer a lightweight device that frees patients from ICU confinement – ultimately to go home – and that is durable enough to provide a long-term solution.”
Device Challenges
Mechanical devices lack the biological sensing features of native lungs and hearts, which autonomically adjust to metabolic changes. To mimic these processes, the team is integrating oxygen, CO2 and pH sensors to detect changes. This information will trigger adjustments to pump speed and ventilation accordingly.
“What we’re really trying to do is offer a lightweight device that frees patients from ICU confinement.”
“Closing the physiologic feedback loop safely – and in real time – is one of the challenges we are trying to solve in the lab,” Ukita said. “But to achieve a truly durable system, we need to develop not only this control and feedback system, but also advanced coating technology, superior gas exchange technology and secure vascular attachments.”
End-stage lung disease patients can also develop pulmonary hypertension, a condition that increases pressure in the lungs and in the right ventricle, overstraining it. They can suffer from a combination of respiratory distress symptoms, inadequate oxygenation of the blood and right heart failure. Without cardiopulmonary assistance, patients with severe right heart failure will die in an ICU, Bacchetta said.
Ovine Study
To optimize a device for patients with right heart failure, they used a right atrium-to-left atrium shunt to bypass both the ventricle and the strained lungs, in essence creating a parallel lung, Ukita said. In respiratory-only applications such as COPD, however, the device will support the lungs without bypassing the right ventricle.
Until now, ambulation with pump-oxygenator circuits has only been demonstrated in healthy sheep. In the recent study, the six ovine subjects had chronic right heart failure. All were fitted with a portable pump-oxygenator circuit attached under the right-atrial-to-left-atrial shunt configuration. Three of the subjects survived to the seven-day endpoint for circulatory support. Two freely ambulated.
“A 50% completion rate was not surprising in this complex of a study,” Ukita said. “Only one failure was tied to device malfunction, with the two others linked to the animals’ sensitivity to surgical and disease-induced stress.”
The authors concluded that the device provided stable respiratory support, resulted in minimal clotting, and enabled stable gas exchange, blood flow and hematologic parameters.
Optimization
Blood biocompatibility – especially hemolysis – remains a hurdle to optimization, Bacchetta said.
Future designs will refine the pump and the cannula to reduce blood damage and clotting. The team is also addressing the bleeding risk of anticoagulants and collaborating with colleagues at other centers to optimize components of the device.
“We have different applications of this technology for different disease-states, but they all require similar feasibility and efficacy testing before we go into human testing,” Bacchetta said. “We’re working on submissions to the FDA.”
Further optimization of surgical technique is also needed to facilitate left atrium access and make it less invasive. Other refinements will include adjustments to cannulation strategy and mechanical support technology to facilitate exercise, Bacchetta said.
Parallel Advancements
Beyond chronic disease applications, the team is working to improve support for wounded soldiers with acute lung injuries in an initiative with the Department of Defense and the Defense Advanced Research Projects Agency.
“These are war fighters who have a lung injury that’s more acute, so they just need a device to support them until their own lungs recover,” Bacchetta said.
“These are war fighters who have a lung injury that’s more acute, so they just need a device to support them until their own lungs recover.”
Ukita added that another aspect of the device development involves resuscitation of war fighters suffering from blood loss.
Additional work is expanding the window for transplant and improving the condition of the donor lungs. Bacchetta’s team is developing an ex vivo lung support system that perfuses and rehabilitates compromised donor lungs in advance of transplantation, expanding the donor organ pool.