Electromagnetic navigational bronchoscopy (ENB) is the diagnostic system traditionally used to examine peripheral lung lesions detected on CT scans. However, a new robot-assisted bronchoscopy system is quickly gaining adherents.
While adoption of the robotic bronchoscopy has been rapid, little comparative data have been generated to establish which of the approaches produces higher diagnostic yields. Vanderbilt, like many medical centers, uses both.
To examine potential differences in patient outcomes, thoracic specialists Fabien Maldonado, M.D., M.Sc., and Rafa Paez, M.D., M.S.C.I., at Vanderbilt University Medical Center, devised a pragmatic, single-center randomized controlled trial that took advantage of features already built into their OR scheduling.
Using patient clusters, the team was able to complete a comparison of the ENB and robotic systems in only one year, with outcomes that will be published soon. They entitled their study RELIANT (Robotic versus Electromagnetic bronchoscopy for pulmonary LesIon AssessmeNT).
“To date, this is the only comparative study that includes robotic bronchoscopy,” Maldonado said. “We use both systems at Vanderbilt, and with a high patient volume, we wanted to know the difference – to take better care of our patients. And we needed to know within a reasonable time frame.”
Racing Against Obsolescence
Demonstrating patient outcomes prior to commercialization of a device is not technically required, provided safety and effectiveness appear similar to those currently marketed.
“Today, we have access to these amazing machines, which are probably very helpful. But we never truly had prospective or comparative clinical data showing improved outcomes,” Maldonado said.
Randomized controlled trials are lengthy by nature, he said.
“The problem is, if you do a randomized controlled trial in the usual way, one of the devices may be obsolete by the time you are finished.”
“The problem is, if you do a randomized controlled trial in the usual way, one of the devices may be obsolete by the time you are finished.”
The last trial this team conducted was for a sponsor company and took six years to complete, Maldano said. While by no means suitable for every device comparison, a pragmatic trial design is a way to compare devices in a fraction of the time and figure out the best way to take care of patients.
Opportune Procedure
With over 1.5 million lung nodules identified each year in the U.S., biopsy of lesions is a high-volume procedure. Just a small difference in diagnostic yield between one system or another becomes substantial when applied to a large patient population.
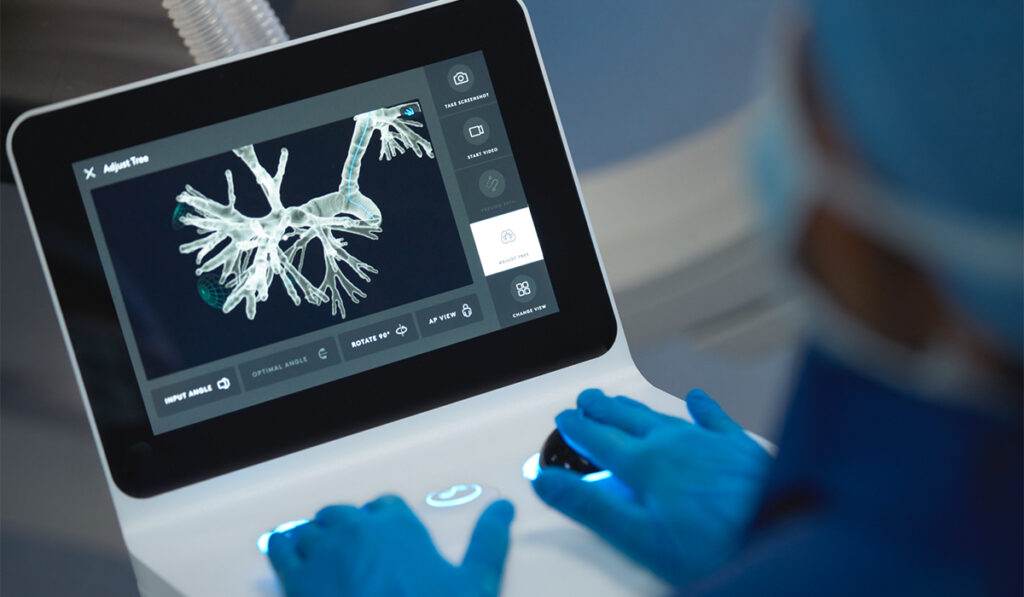
To assess lung nodules, ENB uses low-frequency waves to track a sensor that guides navigation to the lesion of interest. With the recent addition of digital tomosynthesis, which provides three-dimensional imaging capabilities, diagnostic yields of 79 to 83 percent have been reported with ENB.
The robotic platform, called the ION endoluminal system, uses shape-sensing technology to track the position of the catheter within the airways. This approach is believed to provide improved stability and movement precision compared to more traditional bronchoscopes and some consider it easier to master.
Diagnostic yield estimates of 86 percent have been reported, but the evidence is limited. As with ENB, reliance on such data is tainted by the absence of randomization to the two methods. There may also be unknown downsides to robotic bronchoscopy relating to time, cost and radiation exposure that need to be identified.
RELIANT Trial
The RELIANT team enrolled first-time bronchoscopy patients from March 2023 through April 2024. A total of 411 patients had undergone either ENB or RAB.
At Vanderbilt, the interventional pulmonologists were already using both methods on a random basis, depending on the day and the OR.
“We turned what was already random into ‘randomized’ and created clusters, each consisting of an OR day,” Paez said. “All were blinded to the assignment of one or the other system until we opened the daily assignment envelope.
“Because participating in the study would not change the procedure patients were scheduled to receive as they would have without the study, almost all patients agreed to participate and provided informed consent.”
Diagnostic yield was the primary endpoint for the trial. Secondary and safety endpoints included procedure duration and procedural complications.
“Traditionally, in order to adjudicate the primary endpoint of diagnostic accuracy, you needed to wait at least a year after the procedure to see if the benign diagnosis you got was actually benign,” Paez said. “But with diagnostic yield, we were able to adjudicate the primary endpoint within days of the procedure, so we were collecting the data as we went along.”
Using broad inclusion data – of all first-time navigational bronchoscopy patients – also heightened study efficiency, since its design didn’t require numerous variables. Embedding the study as much as possible in existing protocol helped make the study organic.
Greasing the Skids
The team points to support of Vanderbilt’s Center for Learning Healthcare System, whose team were experienced with pragmatic research.
“Without the center’s expertise in things like designing cluster randomization and assuring that we had correct informed consent, outcome descriptions and IRB answers, this might not have happened,” Maldonado said.
Ultimately, the researchers’ goal is to expand to a network of 15-20 centers for multicenter trials.
“You have to find others that have a similar IRB awareness of this approach,” Maldonado said. “But it is an easier sell for other centers when you can show them that the study has already been approved someplace else – in this case, Vanderbilt.”