Featured in the Vanderbilt Eye Institute Impact Report 2024. Peruse the rest of the Impact Report here.
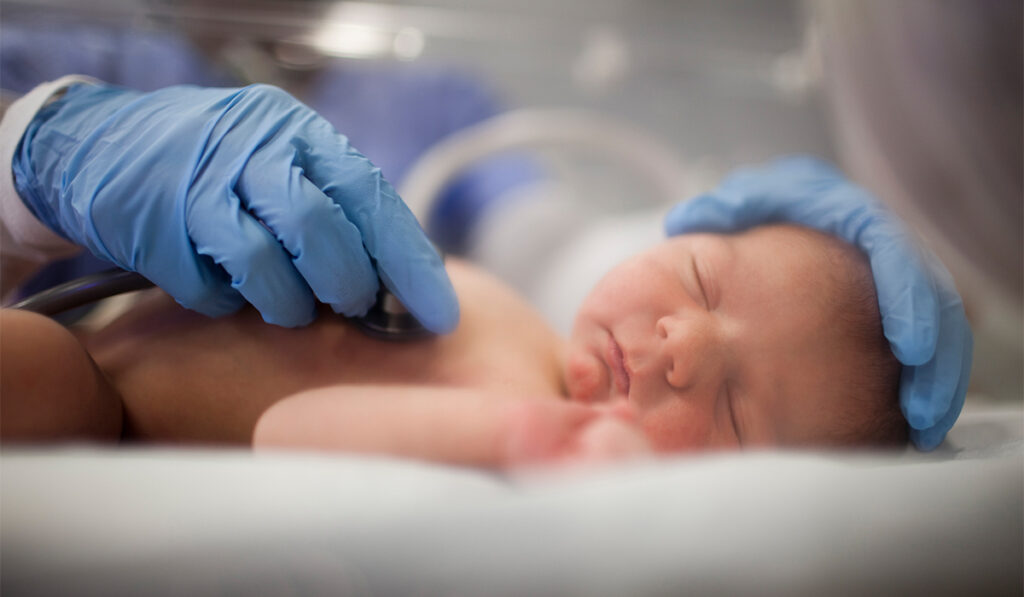
Retinopathy of prematurity (ROP) occurs when abnormal blood vessels grow in the retina. Without prompt treatment, it often leads to lifelong vision disorders — ranging from amblyopia (lazy eye) to blindness.
Common in infants born at 30 weeks or less, ROP affects approximately 14,000 preterm infants in the United States each year. Of those, between 1,100 and 1,500 infants develop into moderate to severe disease that requires medical attention and treatment.
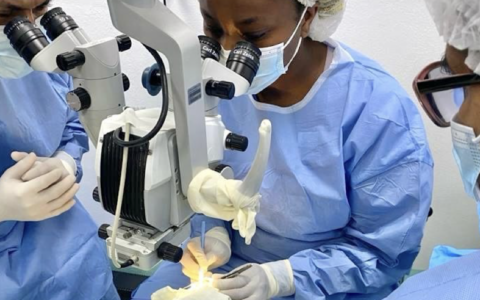
Conventional ROP treatment involves ablation of the peripheral retina, either by cryotherapy or laser photocoagulation, limiting the excessive production of vascular endothelial growth factor (VEGF). Newer treatments include intravitreal injection of an anti-VEGF agent to limit its production.
For over 30 years, Vanderbilt Eye Institute (VEI) has been a leader in ROP research and management, with groundbreaking discoveries in disease pathogenesis, innovations in imaging, and advancements in clinical care.
A recent pilot study led by Irina De la Huerta, M.D., Ph.D., an assistant professor at VEI and a nationally recognized retinopathy specialist, examined the outcomes of a novel protocol that combines intravitreal administration of the drug ranibizumab (Lucentis®), followed several weeks later by laser photocoagulation therapy, in order to balance their independent risks.
Initial data suggest that layering the two modalities could offer retinopathy care teams their most effective treatment yet.
“Each treatment option has offered advantages as well as notable factors for concern,” De la Huerta said.
While monotherapy with an anti-VEGF agent carries a risk of retinopathy recurrence, early ablation halts the normal developmental process of retinal vascularization, potentially resulting in peripheral visual field loss.
“Achieving the best possible outcomes in ROP treatment has been a continued debate,” De la Huerta said. “The key issue has been balancing the effectiveness of a given treatment versus its risk, especially considering that these patients are very young premature infants.”
“The key issue in ROP treatment has been balancing the effectiveness of a given treatment versus its systemic risk, especially considering that these patients are very young premature infants.”
Sequencing Leads to Higher Success
For the initial retrospective pilot study, De la Huerta reviewed cases of 25 premature infants from Monroe Carell Jr. Children’s Hospital at Vanderbilt who were given the combined drug and laser therapy treatment. Each patient had severe enough retinopathy to present a lifelong risk of vision problems.
The average age of patients at their first treatment of ranibizumab was 37 weeks post gestation. Patients received laser therapy five weeks later, on average. In the initial review of the pilot group two years after treatment, none of the patients had experienced a recurrence of their retinopathy.
The guiding principle of the new protocol is its sequencing, De la Huerta explains — combining the two treatments in the optimal order and timing to boost effectiveness and minimize risks. Leading the ROP treatment protocol with the anti-VEGF drug injection permits providers to delay the laser surgery, thus allowing the potential for further normal vascular growth in the retina.
Conversely, the secondary laser treatment following drug therapy cuts down the risk of ROP recurrence. It also supports the use of ranibizumab, which may have fewer unwanted systemic effects but a lower long-term potency than bevacizumab, another drug used to treat ROP.
“In babies treated with ranibizumab, systemic VEGF levels return to normal an average of two weeks after the drug is administered, compared to infants treated with bevacizumab, which suppresses systemic VEGF levels for several months following intravitreal injection,” De la Huerta said. “Low serum VEGF may be harmful to developing organs such as the lung and the brain.”
“In babies treated with ranibizumab, systemic VEGF levels return to normal an average of two weeks after the drug is administered.”
Tracking Development Post Treatment
De la Huerta will continue to track outcomes in the pilot cohort. One key variable will be the children’s ongoing neurocognitive development. Previous research has found that some anti-VEGF treatments for retinopathy of prematurity correlated with children developing emotional and verbal-language ability issues that could surface into school age.
“Down the road, as we continue to track this group, we will be gathering incredibly helpful data to clarify elements of ROP treatment, such as the long-term neurocognitive outcomes that require at least three years of follow-up,” De la Huerta said.
Currently, the research is expanding to follow 200 more Vanderbilt patients receiving the combined protocol. This larger cohort has not yet reached a two-year study mark to identify whether participants are attaining the same strong outcomes as the pilot group.
De la Huerta is hopeful: “This protocol is a culmination of years of study of the risk of ROP recurrence after each type of anti-VEGF treatment and the risk of systemic VEGF suppression in premature neonates.”