Featured in the Vanderbilt Eye Institute Impact Report 2024. Peruse the rest of the Impact Report here.
Regeneration or genetic repair of the retina might one day treat or even cure blinding eye injuries and diseases, from age-related macular degeneration to diabetic retinopathy.
Yet, the adult retina is a delicate and notoriously difficult tissue to study due to the tendency of its cells to die off within days of being placed into culture for research.
The prevailing approach is to use tissues created from stem cells or very young animal tissue for laboratory studies. These advances are promising, but they have limited value for adult retinal research because they fail to model the retina’s mature state of development accurately.
Developing conditions for sustained tissue survival in the laboratory prepares the way for early-stage drug testing, cell reprogramming, tissue regeneration, and disease modeling.
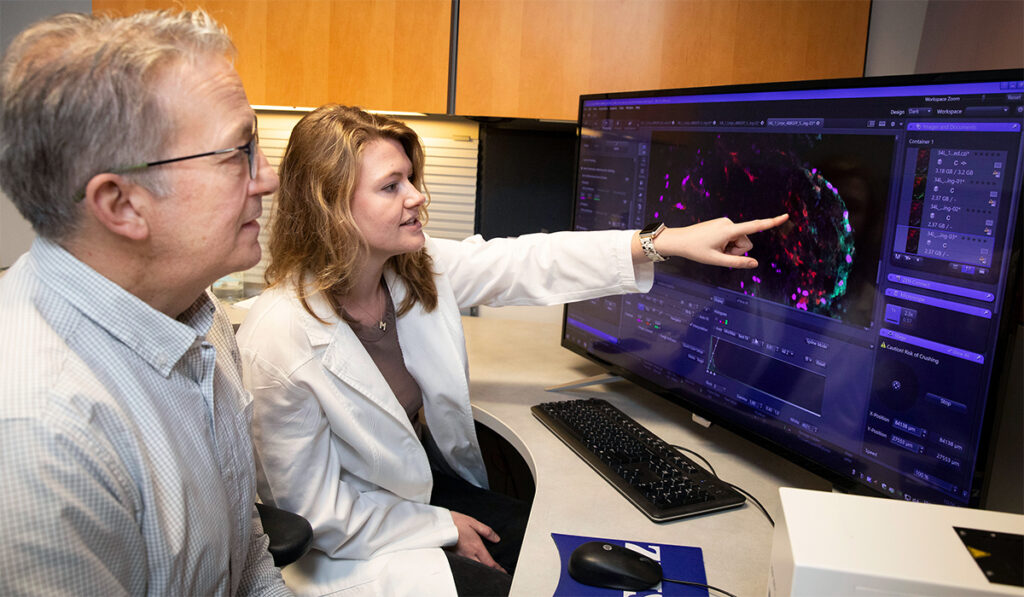
“This is most certainly a high-risk, high-reward area for discovery,” said Edward M. Levine, Ph.D., the William A. Black Professor of Ophthalmology and a professor of cell and developmental biology at Vanderbilt University Medical Center. “If we can establish a platform to cultivate adult tissue for controlled study, it will change the game for retinal disease research and open a much wider door for new, gene-focused therapies.”
The Levine Laboratory for Retinal Development and Regeneration at the Vanderbilt Eye Institute is known worldwide for advancing the science of retinal development and specific genetic interventions that could stimulate the retina into curative regeneration. Over nearly two decades spent building this legacy, the lab also has become a leader in innovating the practice of laboratory research.
“It’s exciting to report that, at the moment, we are testing several promising innovations that could bring retinal disease research much closer to solving its fundamental problem of reliably using adult tissue cultures,” Levine said.
Redesigning a Key Tool
One of these innovations employs electroporation, a broadly used laboratory process that allows researchers to inject a substance such as DNA or a signaling molecule into cells through an electrical current. However, when applied to retinal tissue the traditional electroporation approach poses a problem, Levine noted.
The tissue needs to be submerged in a liquid to allow current flow, but the adult retina is cultured at the boundary between the air and the liquid growth media, and sustained submersion quickly deteriorates the sensitive tissue.
In pursuit of an idea envisioned by predoctoral fellow Megan Stone, Levine’s team is developing an alternative method of electroporation that uses an “air-liquid interface,” a novel gel-based system that avoids submerging the cultured tissue in liquid media or placing it into potentially damaging direct contact with the electrode.
“We want to control the quality of the tissue instead of letting it degenerate on its own,” Levine said.
“If we can establish a platform to cultivate adult tissue for controlled study, it will change the game for retinal disease research and open a much wider door for new, gene-focused therapies.”
The team recently reported its findings showing that its alternative electroporation method successfully transferred the research gene into the Müller glia of adult mice and preserved the integrity of the retinal tissue. They focused on Müller glia cells due to their widespread role in the retina and their potential to one day be used to stimulate neural regeneration in humans.
Additionally, it appeared that the successful application of the electrical current alone stimulated an increase in Müller glia.
“We’re culturing the animal retina on a filter with media below,” Stone explained. “The retinas adhere to that filter and can take up nutrients from the media, which forms a very thin film around the tissue. We then take a piece of gel, adhere that to an electrode, and make a pseudo-column through which we deliver DNA into the tissue.”
If the new platform continues to prove effective, it could have an even broader impact than its immediate potential for advancing eye disease research, Levine said.
“This method of electroporation requires no specialized equipment and is inexpensive. It could be easily adapted to study other tissues in which air-liquid interface cultures are used, such as the lung, intestine, skin or brain.”
For her work, Stone was awarded a Ruth L. Kirschstein National Research Service Award from the National Institutes of Health, which is given to the most promising predoctoral students in the U.S. The award provides salary support and additional funds to support career development for three years.
Innovations Inform Retinal Science
The Levine lab’s intertwined approach to advancing tools and techniques concurrent with scientific theory is a unique facet of its culture.
In addition to the new retina model, the lab has a considerable portfolio of innovations entering experimental use now or on the near horizon, Levine says. As it enters its 17th year of NIH funding, the lab also has made significant discoveries that advance retinal science, such as testing specific genetic interventions that restore the formation of the retina and eye in animal models to mimic congenital birth defects found in human eyes.
“In order to achieve scientific and ultimately therapeutic advances in a field like retinal regeneration, we have to constantly improve the methods and tools available to take measurements, collect data, and adjust the conditions of tissue cultures,” Levine said.
Among the team’s evolving proposals is a multi-component system that will allow researchers to make real-time measurements of changes in tissue cultures, rather than waiting to perform analyses at the close of experiments.
The system would leverage one of the most advanced imaging technologies available today — structured illumination microscopy — in combination with several diagnostic devices now used in other medical applications. A unique collection of laboratory mice whose retinal cells glow under certain types of light will make visualizing changes much more accessible, Levine said.
“These new models will help us and other researchers better understand the pathology of retinal injury and proliferative vitreoretinopathy, situations that best reveal the complexity of the barriers to regeneration. That would bring another level of robustness to the research.”