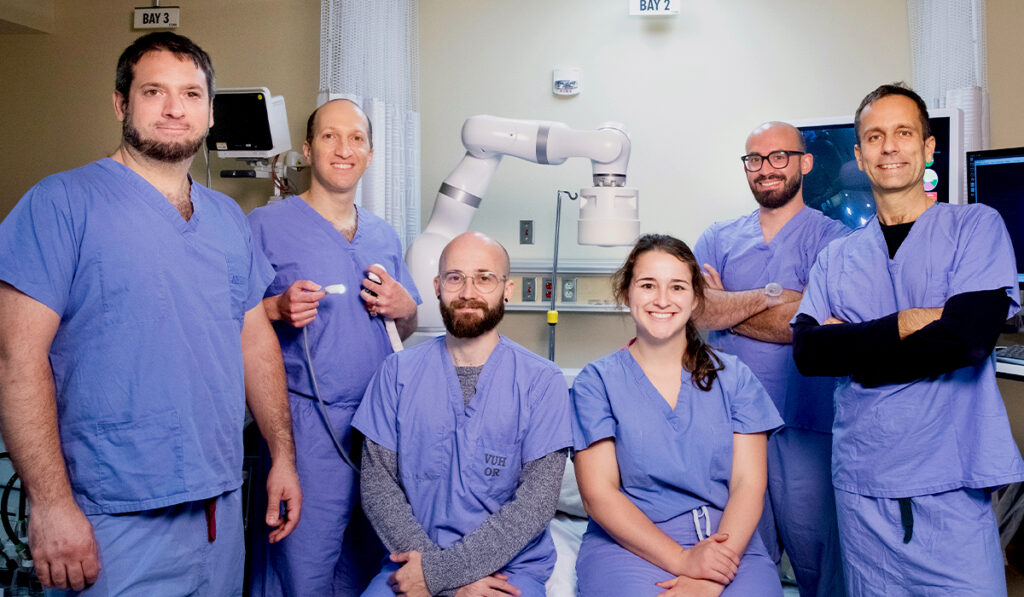
In the second installment of Discoveries in Medicine’s two-part interview with Keith L. Obstein, M.D., co-developer of a robotic colonoscopy system, he maps the path to the marketplace.
In our previous interview, Obstein discussed the design he helped conceive and development of the system, including its advantages over conventional colonoscopy. Read part one of the series here.
Launching a Startup
Discoveries: You’ve gone from concept into development of a feasible colonoscopy system. What lies ahead before it can be introduced to patients?
Obstein: In collaboration with Dr. Pietro Valdastri, and an experienced team of experts and serial entrepreneurs, we formed Atlas Endoscopy, a tech company focused on commercializing the platform. The vision of the company is ‘colonoscopy democratized.’
An essential part of the company’s mission is to expand access and increase capacity in health care systems. In the face of high demand for colorectal cancer screening, the health care system is unable to meet the current needs and warrants a response.
“There are healthcare deserts across the country where colonoscopy services are not accessible. Imagine if high-quality endoscopic care could be carried out in rural or remote regions where access to these specialized services does not exist or is limited.”
We are still in the early days of our clinical trials, but this new system is incredibly exciting and showcases where the field is heading. We’re actively taking steps needed for FDA approval and, in the future, we plan to bring it to market in the United States.
There are healthcare deserts across the country where colonoscopy services are not accessible. Imagine if high-quality endoscopic care could be carried out in rural or remote regions where access to these specialized services does not exist or is limited.
If the technology were widely implemented, we could potentially take colonoscopies outside of traditional surgical or endoscopic settings, democratizing access.
There is also the potential to bring the procedure directly to the patient even, in the comfort of their own residence, rather than the patient having travel to other surgical care settings.
Discoveries: What are your funding sources and what resources do you hope will support you as you go to market?
Obstein: We have been fortunate to obtain funding from internal sources, agencies in the United Kingdom, and from the NIH. We are also thankful to the Brock Family Center for Applied Innovation for their important contribution to advancing novel platforms at Vanderbilt. In the future, we are looking to license the technology, as Vanderbilt owns the intellectual property for the system.
Looking to the Future
Discoveries: How is the system designed to support the integration of artificial intelligence (AI) software in the future?
Obstein: The hardware and overall architecture of the system was designed to support additional software add-ons. These include AI applications. Even at this time, one could use commercially available, computer-assisted polyp detection software with the MFE.
“We recognized the potential for AI to transform medicine early on and have accounted for it in the design of the platform. In essence, the platform is inherently future-ready.”
The diagnostic and therapeutic applications stemming from AI are vast. We recognized the potential for AI to transform medicine early on and have accounted for it in the design of the platform. In essence, the platform is inherently future-ready.
Discoveries: What are some of its other built-by-design features?
Obstein: The magnetic flexible endoscope is single-use and disposable, eliminating the need for costly repairs, maintenance contracts, or reprocessing. Additionally, with a disposable endoscope, you significantly lower or potentially eliminate the risk of infection and cross-contamination.
The magnetic flexible endoscope platform was also designed to minimize procedure variability – ensuring that every patient has a high-quality exam irrespective of their geographic region.
Discoveries: When do you anticipate the system will be widely available?
Obstein: With the correct funding and partners, I think a five-year time frame would be reasonable. As part of our current NIH grant, we plan to proceed with the next part of our first-in-human clinic trial focusing on patients with inflammatory bowel disease. Concurrently, Atlas has licensed the technology and is now navigating their own path toward FDA approval.
Recognizing the Team
Discoveries: Who do you want to acknowledge for their work on this project?
Obstein: A huge debt of gratitude goes to my long-time collaborator Dr. Pietro Valdastri and to the numerous graduate students who have been instrumental in the success of the platform. I would also like to thank the Vanderbilt Institute for Surgery and Engineering for their continued support and all of the funding agencies who supported this project. Without everyone on the team, we would not have made it to where we are today.