After decades of experimentation, researchers at Vanderbilt University Medical Center have demonstrated that inhibition of the serotonin 2B receptor (5-HT2B) has a powerful potential to alleviate suffering following myocardial infarction and in patients with pulmonary arterial hypertension (PAH), a rare but fatal disease.
W. David Merryman, Ph.D., the Walters Family Chair of Biomedical Engineering, and Craig Lindsley, Ph.D., William K. Warren Jr. Chair in Medicine and director of the Warren Center for Neuroscience Drug Development at Vanderbilt, have led the charge in not only establishing evidence but also in developing serotonin 2B antagonists.
“The first drugs we used worked in our models, but in humans, these would have serious neurological side effects,” Merryman explained. “Then we formulated a targeted drug that doesn’t cross the blood-brain barrier. At that point, we knew we now had a path to human trials.”
“We formulated a targeted drug that doesn’t cross the blood-brain barrier. At that point, we knew we now had a path to human trials.”
While this molecule has the potential to limit fibrotic scarring after myocardial infarction – and impact more pathologies – the first target was PAH treatment.
“Testing the molecule in the more homogenous population of PAH was the best way to go. It would have been very difficult to design a test that controlled for all the variables in myocardial infarction,” he said.
They are now preparing for the first ever PAH human trial of this inhibitor to test its safety, tolerability, and eventually, effectiveness in patients with active PAH.
Targeting PAH Mechanisms
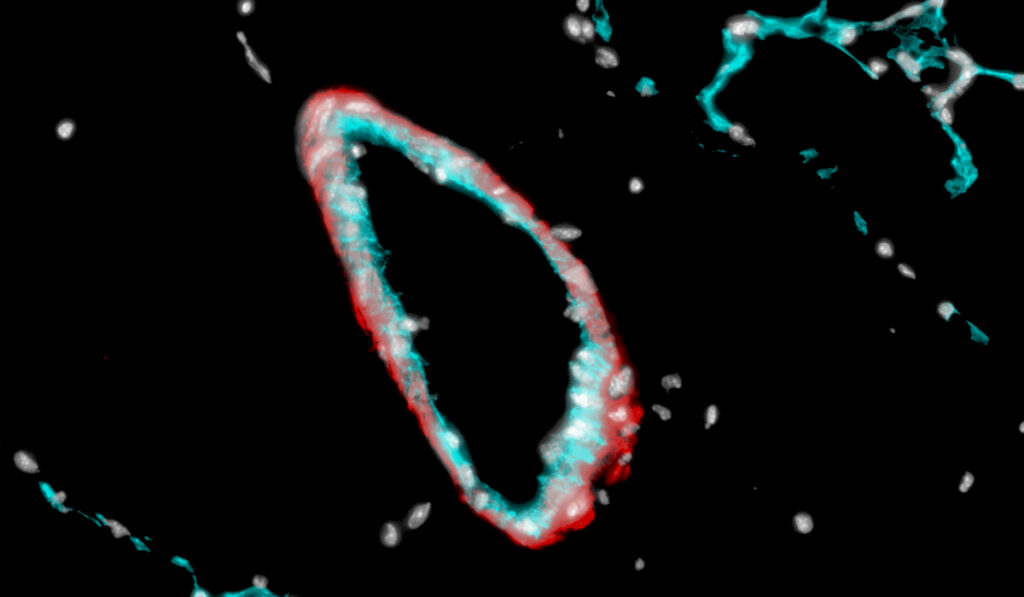
As early as 2016, Merryman and colleagues determined that serotonin 2B receptor signaling is complicit in the arteriole damage found in PAH, in which proangiogenic cells (PACs) produced by bone marrow enter the circulatory system, occluding the lung microvasculature and aggravating the lung lining leading to scarring and narrowing.
The resulting lung pressure has downstream effects on the pulmonary artery wall, which becomes muscularized and stiffened. In turn, this phenomenon puts extraordinary pressure on the right ventricle, causing right heart failure. Patients with PAH typically live around 10 years after diagnosis.
“Our data indicate that bone marrow-derived PACs are necessary for the development of experimental PAH in the setting of endovascular injury, and they likely contribute to small artery stiffening and remodeling,” Merryman said.
He and his colleagues found that serotonin 2B signaling is a necessary component of this PAC recruitment and the havoc it wreaks. By inducing PAH in mice, and then blocking the serotonin 2B mechanism in the PACs to prevent them from invading the lungs, the researchers found they could prevent and even reverse damage.
Taming Left Heart Remodeling
Merryman says that following trials, the antagonist’s use for treating myocardial infarction could become an off-label yet important application.
In their mouse models, the researchers saw that the same serotonin 2B signaling also triggers post-myocardial infarction fibrotic scarring and remodeling in damaged hearts.
Following a heart attack, cardiac myofibroblasts activate to repair blood vessel ruptures and areas of myocardial wall damage. These fibrotic “repair crews” deposit thick layers of collagen to patch the compromised area and then extend the process beyond the border of the lesion, affecting adjacent areas, Merryman said.
“It’s a hypertrophic response that affects uninjured cardiomyocytes,” he added.
The result is a stiffer myocardium and lower ejection fraction, creating a path for heart failure.
The Inhibitory Difference
Serotonin 2B antagonism resulted in collagen fiber redistribution to thinner collagen fibers that were more anisotropic. This enhances left ventricular contractility, decreases fibrotic tissue stiffness, and limits the hypertrophic response of uninjured cardiomyocytes, Merryman said.
In mice with serotonin 2B signaling inhibited or genetically altered, the heart had a 50 percent greater ejection fraction six weeks after heart attack than in unaltered mice. Further, echocardiography revealed that early inhibition led to a functional scar that was less likely to expand beyond the initial wound.
“We found that the drug models have an acute and long-term effect,” Merryman said. “If we can stop the expansion in that first week, we could probably preserve the healthy heart tissue around the damaged area.”
Route to Market
Merryman says following the upcoming trials, PAH will be the primary indication for the drug. At-risk patients might be identified by single nucleotide polymorphisms that would indicate potential value in targeting serotonin 2B. Patients with active PAH also may benefit, which he says is a remarkable prospect.
Once the treatment is available, the patient would take the drug over the week following the heart attack, during the heart remodeling phase.
“We’ve done a lot of screening and testing of the compounds , and we hope we’re one year away from testing this investigational new drug in humans,” Merryman said.