Researchers at Vanderbilt University Medical Center have shown for the first time that when one optic nerve in the eye is damaged, as in glaucoma, the opposite optic nerve comes to the rescue by sharing its metabolic energy.
In doing so, however, the undamaged optic nerve becomes vulnerable to further metabolic stress, which could explain why the neurodegeneration observed in this and other diseases spreads between brain regions.
The report, published in the Proceedings of the National Academy of Sciences, suggests a potential new approach to treating neurodegenerative diseases like glaucoma and Alzheimer’s disease.
“This implies that a way to slow neurodegeneration generally would be to boost metabolic resources of the involved neurons,” said David Calkins, Ph.D., director of the Vanderbilt Vision Research Center. Calkins is senior author of the study.1
“This implies that a way to slow neurodegeneration generally would be to boost metabolic resources of the involved neurons.”
The Role of Astrocytes
Calkins and colleagues have previously shown neurodegeneration in glaucoma involves an early axogenic response that counters intraocular pressure-related stress, slowing progression and maintaining signaling to the brain. However, in reviewing the ophthalmology literature they observed descriptions of “strange bilateral effects” – treatment of degenerative disease in one eye only would cause improvement in the other eye. “There were examples from macular degeneration, glaucoma, retinal transplant – even in gene therapy to cure Leiber’s neuropathy,” Calkins said.
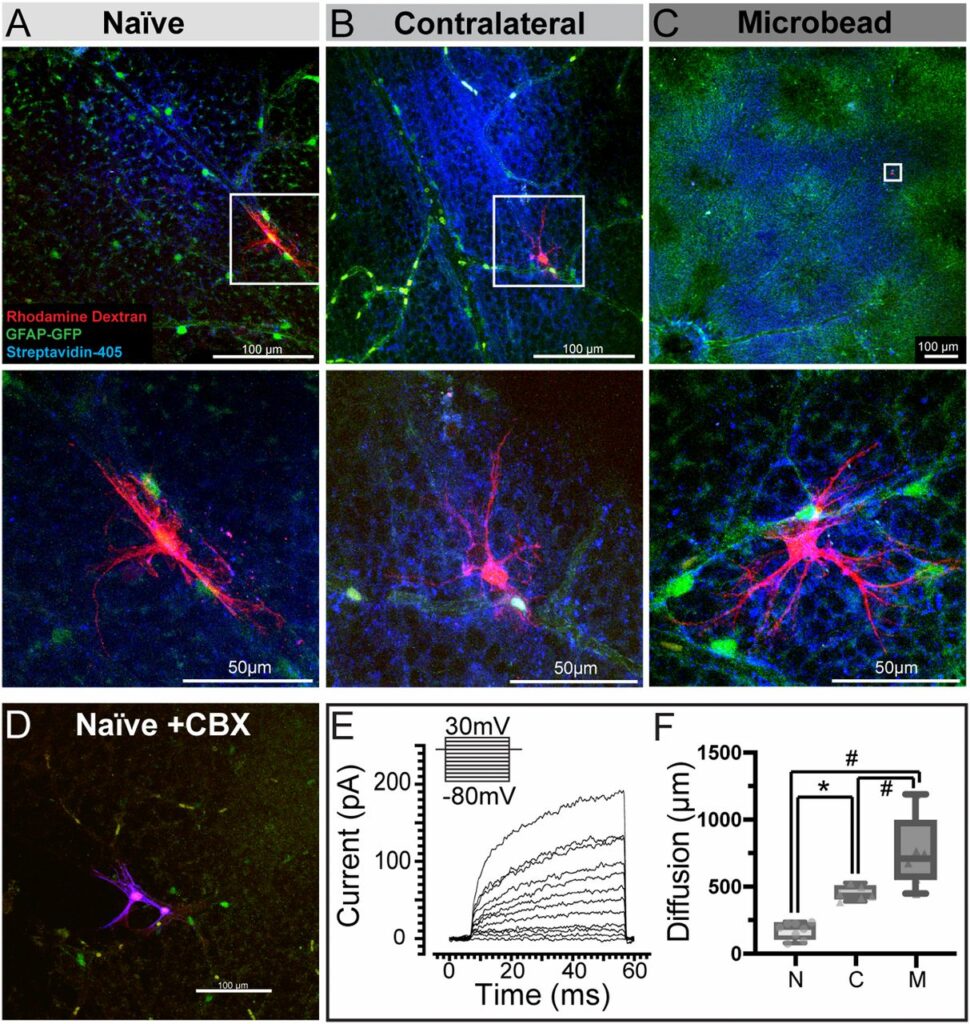
The researchers were also studying astrocytes, the star-shaped glial cells that store glycogen and release it as glucose, and how the cells are able to store energy while neurons cannot. Astrocytes, they hypothesized, are the fuel neurons need to function, and could form a large network that would essentially connect the neurons in one eye to the neurons in another.
Mapping Metabolic Activity
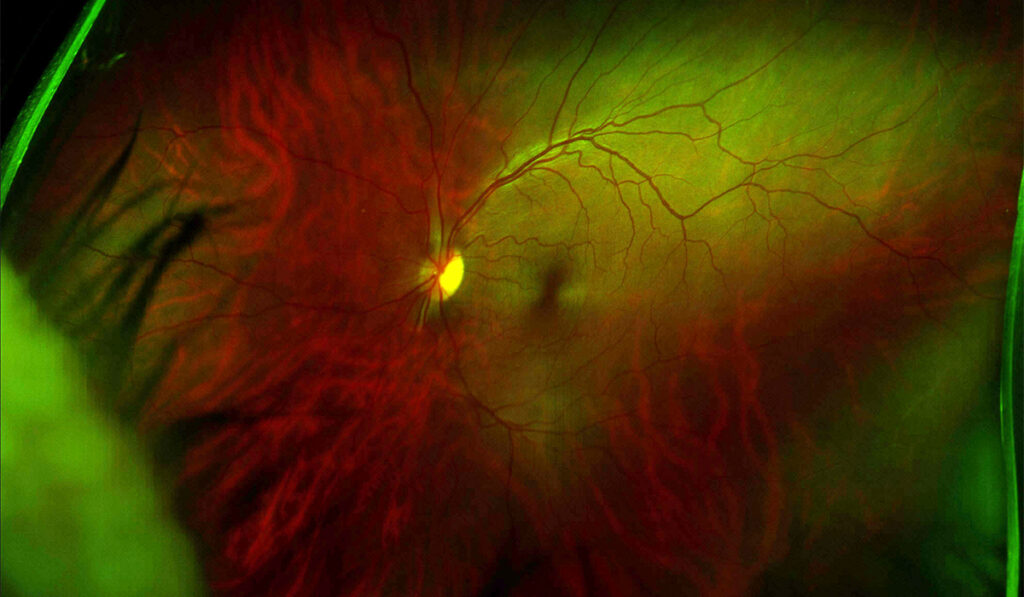
Using positron emission tomography (PET) imaging to map the metabolic activity of cells in different tissues, the authors showed that when one optic nerve was stressed by a rise in intraocular pressure, metabolites including glycogen were transferred from the healthy optic nerve via their crossover point in the brain.
“The energy is transferred up one optic nerve, across the optic chiasm in the brain and back down to the other eye, which is a tremendous distance for metabolites to travel,” Calkins said. “We don’t know exactly how it’s done.”
“The energy is transferred up one optic nerve, across the optic chiasm in the brain and back down to the other eye.”
The transfer, the group discovered, is dependent on connexin 43 (Cx43), the protein that makes up the gap junctions in astrocytes, and which permits the exchange of small molecules between them. When Cx43 was genetically knocked out in a mouse model, the energy transfer did not happen within astrocyte networks between the two optic nerves.
Boosting Astrocyte Capacity
The resource transfer phenomenon helps explain bilateral effects seen in neurodegenerative diseases like Alzheimer’s disease, which can start in one hemisphere of the brain and travel to the next. While sharing energy helps the diseased tissue, the tissue donating its energy stores becomes more susceptible to subsequent injury. “There’s a price to be paid,” Calkins said.
The next phase: increase the capacity of astrocytes to hold energy and transfer it more efficiently to the nerves in the brain. Calkins says using gene therapy to reprogram neurons in certain diseases of the visual system has been shown to be effective.
“One way that metabolic resources can be boosted is by targeting astrocytes to create and store more metabolites to share with neurons. What we’re trying to do now is reprogram astrocytes by using viruses to insert genes into their DNA.”