Nicotine is an enticing new treatment for psychiatric and cognitive disorders. For Paul Newhouse, M.D., director of the Center for Cognitive Medicine at Vanderbilt University Medical Center, it’s been the focus of his career.
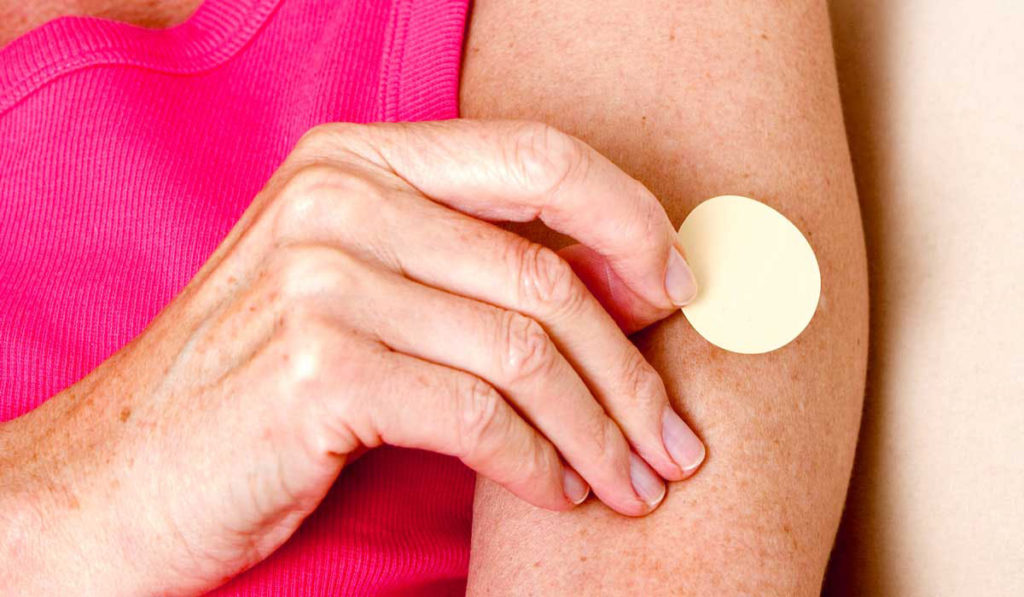
As far back as 1988, Newhouse demonstrated intravenous nicotine could hold promise to treat Alzheimer’s. Now, he’s spearheading the Memory Improvement through Nicotine Dosing (MIND) Study that focuses on transdermal nicotine patches as a viable treatment for people at risk of Alzheimer’s.
“I’m excited that we get to test this approach in a big way,” Newhouse said. “It’s inexpensive, readily available, and potentially over-the-counter. It is not a drug that’s going to cost $100,000 a year, or take 10 years to get to market. If it works, it could be immediately available.” Nicotine treatment for Alzheimer’s disease shows promise among patients in the test study.
Mechanism of Action
Newhouse and others have repeatedly shown activating nicotinic receptors in the brain can improve cognition. These receptors are gradually lost during Alzheimer’s. Current Alzheimer’s medications aim to increase levels of neurotransmitters that activate the receptors, thereby boosting their effectiveness.
Transdermal nicotine activates the receptors more directly. Said Newhouse, “Oddly, a naturally occurring substance that we associate with bad outcomes like smoking, if used in a different way, may turn out to be helpful for patients with memory loss.”
Scaling Up
In a recent pilot trial, Newhouse tested whether transdermal nicotine might help treat cognitive decline associated with breast cancer chemotherapy. Participants in the study reported cognitive improvements, though the sample size was small (25 women).
Another pilot tested the approach in 74 people with mild cognitive impairment that often precedes Alzheimer’s. A six-month, transdermal nicotine intervention improved cognitive performance across those who received it.
Newhouse plans to enroll 300 participants in the MIND study. Men and women at more than 35 study sites will wear a transdermal nicotine (or placebo) patch daily. The daily nicotine dose will titrate up to 21 mg in the first six weeks, and remain at 21 mg for nearly two years, with a taper in the final month.
A Focus on Alzheimer’s
All of the participants will be age 55 to 90 and experiencing mild cognitive decline – people most at risk of developing Alzheimer’s. The researchers will use a battery of tests to assess participant cognition before and after the intervention.
Said Newhouse, “We’re going to look for the impact on their attention, their memory function and even their brain structure.”
“We’re going to look for the impact on their attention, their memory function and even their brain structure.”
The tests will include seven evidence-based measures that identify changes in dementia, Alzheimer’s, depression, recall and other cognitive symptoms. The researchers will also collect cerebral spinal fluid samples and volumetric MRI from each participant at baseline and 25 months. They will look for changes in established biomarkers associated with Alzheimer’s.
Other Applications
Newhouse hopes nicotine could be used to treat a range of cognitive diseases. He’s studied the role of nicotine in mood, attention-deficit hyperactivity disorder, central nervous system degenerative diseases (like Parkinson’s), Alzheimer’s and others.
In one transdermal nicotine study, over half of participants reached clinical remission for their late-life depression. Newhouse is also leading trials to test transdermal nicotine in people experiencing cognitive decline associated with Down syndrome.
“This will not be the only solution to cognitive disease, but it could be part of the solution,” Newhouse said. “It could make a difference even it pushes the disease off just a few years.”