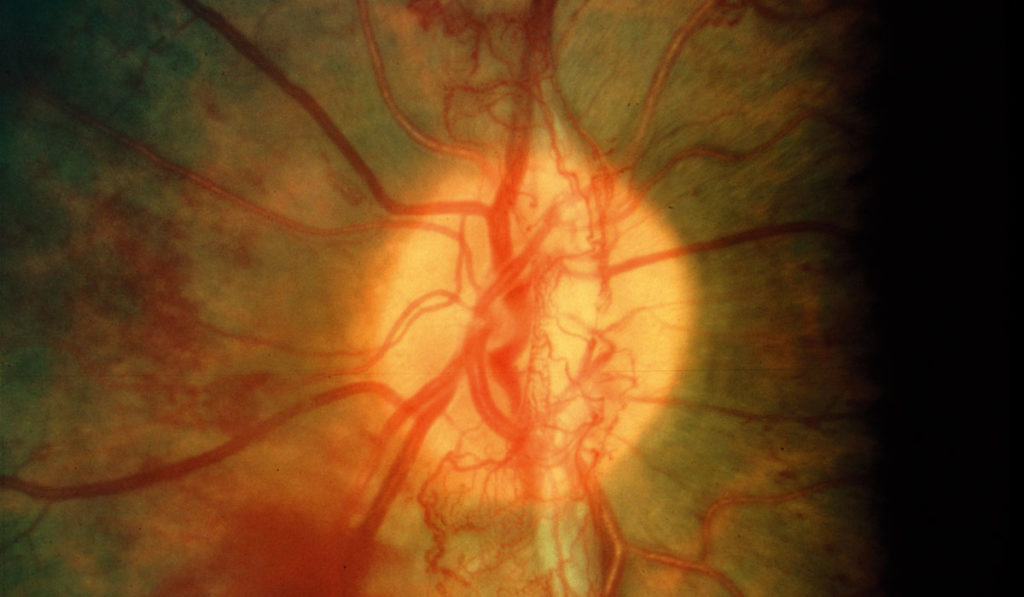
Neovascularization (NV) caused by hypoxia is the major cause of vision loss in proliferative retinopathies such as retinopathy of prematurity, diabetic retinopathy and retinal vein occlusion. While technologies for imaging the retina have been significantly improved through advances in instrumentation, a non-invasive imaging method has not been available to predict NV onset, track progression or monitor treatment response.
Novel molecular imaging technologies developed at Vanderbilt University Medical Center are set to address this resource gap. Imam Uddin, Ph.D., assistant professor of ophthalmology and visual sciences and biomedical engineering, in collaboration with colleagues at the Vanderbilt Eye Institute, has developed HYPOX-4, a novel fluorescence imaging probe enabling the detection of retinal hypoxia in living animals. On a parallel track, the team has developed nanotechnologies to detect mRNA transcripts upregulated in response to hypoxia or inflammatory cytokines.
“These techniques allow us to image neovascular changes in preclinical animal models,” said Uddin. “That capability had not existed before.”
“We’ve been studying the mechanisms of neovascularization for years. Now we may have the ability to predict its onset and track its progression at the molecular level.”
“We’ve been studying the mechanisms of neovascularization for years,” said John Penn, Ph.D., Snyder Endowed Chair in Ophthalmology and Visual Sciences at Vanderbilt. “Now we may have the ability to predict its onset and track its progression at the molecular level.”
Detecting Hypoxia in a Mouse Model
Earlier versions of the HYPOX probe (HYPOX-1, -2, and -3) had allowed for ex vivo hypoxia detection, but their application to in vivo imaging was limited due to poor pharmacokinetic characteristics. To achieve the goal of in vivo imaging, Uddin and colleagues continued efforts to develop a probe having the correct pharmacokinetic properties to allow enhanced diffusion into capillary-free retinal tissue.
Through the hypoxia-induced fluorescence of HYPOX-4, the investigators were able to detect retinal hypoxia in a mouse model of retinal vascular pathology. This was the first report of real-time hypoxia imaging in the retina of living animals by a fluorescence-based method.
“HYPOX-4-mediated retinal imaging is non-invasive, which makes it an excellent tool for diagnosing and for monitoring retinal hypoxia during disease progression or in response to therapy,” Uddin said.
Predicting Neovascularization with Nanoparticles
Once they achieved a method for detecting hypoxia in vivo, Uddin and colleagues began looking at mechanisms for predicting NV in retinopathy. They focused their efforts on the use of vascular cell adhesion molecule 1 (VCAM-1) as a predictive biomarker. Elevated levels of VCAM-1 have been reported in a number of human retinal diseases, therefore detection of VCAM-1 may be used to dissect molecular mechanisms in preclinical studies.
“We believe that these biomarkers are taken up by bone marrow-derived, bloodborne macrophages,” said Uddin. “Our hypothesis is that these cells migrate to the retina; we want to know why they’re migrating and what their role is once they get there.”
The primary strategy is based on hairpin-DNA functionalized gold nanoparticles (hAuNP), which are biocompatible gold nanospheres engineered to enter living cells and fluoresce upon association with target messenger RNA (mRNA) or microRNA transcripts. In preclinical experiments, hAuNPs were used in cultured retinal cells to image disease-relevant biomarkers and in mouse models to characterize their biodistribution and safety profiles.
“The ability to image hypoxic cells can enable researchers to track this critical hallmark of retinal disease and to understand how retinopathies develop,” Uddin said.
RNA Sequencing to Detect Change
In a new study to be published later this year, Uddin is working with Vanderbilt Technologies for Advanced Genomics (VANTAGE) to evaluate single cell RNA sequencing data of mononuclear cells isolated from whole blood. RNA has never been imaged in a living system of retinopathy.
“When a tissue is stimulated to change, the first thing it does is amplify messenger RNA which creates new proteins,” said Uddin. “In our most recent study, a new cluster of cells appeared in animal models of retinal hypoxia that is not present in normal animals. If hypoxia can be detected before neural or vascular damage, it could perhaps aid the physician in determining when to begin therapeutic intervention.”
“Being able to detect or even predict disease progression in premature infants and diabetic patients could help to preserve vision in millions of patients worldwide.”
“These new technologies could have immediate translational impact,” said Penn. “Being able to detect or even predict disease progression in premature infants and diabetic patients could help to preserve vision in millions of patients worldwide.”