Jay Bhave, M.D., began his research career at Vanderbilt University Medical Center working with Billy Hudson, Ph.D., on Hudson’s pioneering studies of Goodpasture’s disease and Alport syndrome. Hudson’s research has been instrumental in clarifying the function of the type IV collagen network in structuring and regulating basement membranes, including the glomerular basement membrane (GBM).
Bhave, now leads a Vanderbilt laboratory that is furthering research into the composition of the GBM and surrounding extracellular matrix. Bhave hopes this research could generate new therapies for rare and common kidney diseases.
“The GBM is one of the major locations for nearly every kidney disease,” Bhave said. “That includes the cells that interact with that basement membrane or the nearby mesangial matrix. The whole apparatus is interconnected, so you can’t study the GBM in a vacuum.”
In addition to its role in Goodpasture’s disease and Alport syndrome, dysfunction in the collagen IV network may be an early pathogenic step in the development of diabetic kidney disease.
“A rare disease like Alport syndrome gives us a more simplified system to isolate and figure out how the collagen IV network works.”
“A rare disease like Alport syndrome gives us a more simplified system to isolate and figure out how the collagen IV network works,” Bhave said. “With more information about specific types of collagen IV in the GBM and how they’re assembled, we can investigate how the collagen IV network is dysregulated in a very complex disease like diabetic kidney disease.”
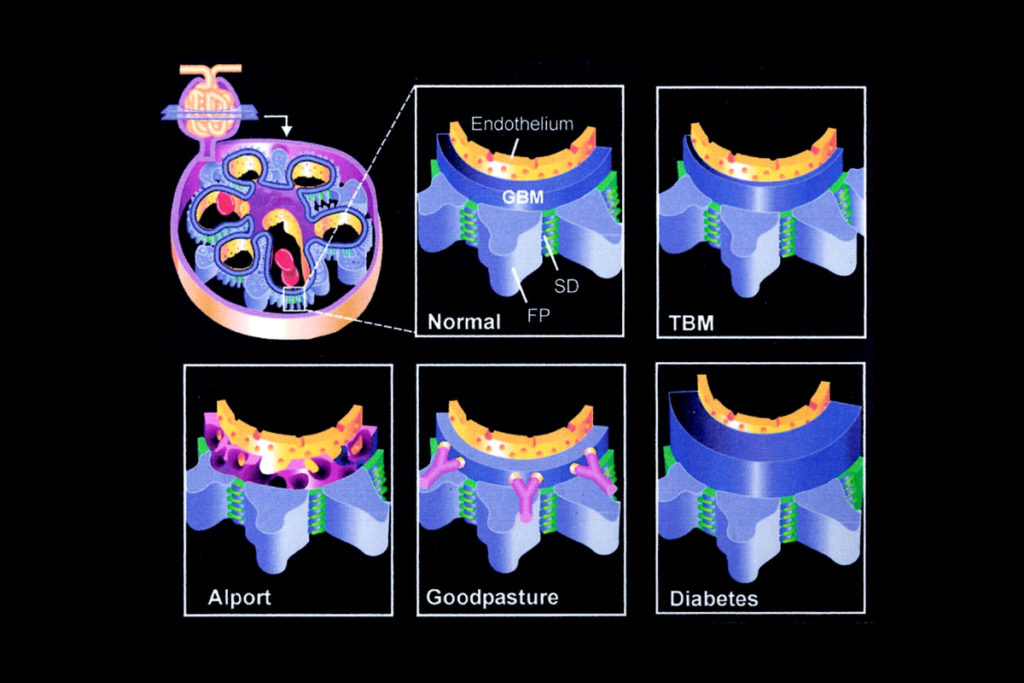
Collagen IV Degradation in Goodpasture’s Disease and Alport Syndrome
In Goodpasture’s disease, a rare autoimmune disease, the immune system creates antibodies against the collagen IV network. If not treated quickly, the resulting inflammatory process leads to destruction of the kidney glomerulus. Goodpasture’s disease can also attack the collagen IV network in the alveolar basement membrane in the lung, at times leading to fatal pulmonary disease.
“Dr. Hudson did some of the pioneering work on Goodpasture’s disease,” Bhave said. “He discovered where these antibodies actually bind to the collagen IV network and protein, giving insight into how this network is formed at a very molecular level.”
In Alport syndrome, a genetic condition, the GBM develops without the collagen IV network, thereby lacking its mesh-like structural reinforcement. As the condition progresses and the GBM tears, damage progresses to the rest of the glomerulus and the filter begins to fail, evidenced by protein in the urine and loss of kidney function. In most patients, Alport syndrome will lead to end-stage renal disease requiring transplantation or dialysis—as early as the teenage years.
Collagen IV and Diabetic Kidney Disease
With diabetic kidney disease, changes to the GBM look very different from the attrition seen with Alport syndrome. Even prior to the onset of diabetic nephropathy, the collagen IV network within the kidney begins to thicken. Much of this thickening occurs within the GBM.
“The functional consequence of that thickening is still unclear, and it is a question that we’re researching actively,” Bhave said. “It may just reflect dysregulation of the podocytes that sit on the GBM and are responsible for making and degrading the GBM. The thickening presumably reflects overproduction of the GBM proteins and not enough degradation. We hope that by understanding how this happens, we may be able to develop treatments.”
Lessons about the structure of the GBM and glomerular filtration apparatus may provide insight into how to design an artificial kidney.
In addition to the hope of developing more targeted therapies aimed at the molecular components of the collagen IV network and GBM, lessons about the structure of the GBM and glomerular filtration apparatus may provide insight into how to design an artificial kidney. This includes the work of Vanderbilt physician-scientist William Fissell, M.D., associate professor of Nephrology and Hypertension. “Bill Fissell is doing pioneering work developing the artificial kidney. As part of his basis for designing the artificial device, he is looking at how nature did it with the glomerular filtration barrier,” Bhave said.
Current and Future Research
During his postdoctoral work in Hudson’s lab, Bhave discovered an enzyme known as peroxidasin, which provides essential reinforcement to the collagen IV network by catalyzing development of sulfilimine cross-links.
“This research is interesting for two-fold reasons,” Bhave said. “The collagen IV network depends on peroxidasin to form properly. But if that enzyme is overactive, it may be related to the inability to degrade the collagen IV network that seems to play a part in diabetes. We know that in diabetes this network is not degraded as efficiently. If those enzymes involved in assembly of the collagen IV network could potentially be targeted by drugs, we could prevent the overaccumulation of collagen IV.”
“Besides endowing structural stability and resistance to degradation, sulfilimine cross-links in collagen IV also block the binding of auto-antibodies to collagen IV in Goodpasture’s disease,” Bhave said. “The loss of peroxidasin function and these cross-links may be a set up for developing Goodpasture’s disease and could be a place where we can intervene.”
Bhave’s lab is continuing research into peroxidasin and its role within collagen IV formation and regulation. In addition, Roberto Vanacore, Ph.D., assistant professor of Nephrology and Hypertension, is leading research into another enzyme, lysyl-oxidase-like 2, similarly involved in forming the collagen IV network.
“There are very few kidney diseases that don’t involve dysfunction in the GBM either directly or through the network of cells that interact with it,” Bhave said. “Learning more about that system will, hopefully, translate into treatment for the majority of kidney diseases that afflict patients in the U.S.”